One of the most important generalizations in immunology the end of the 20th and the beginning of the 21st centuries. was the creation of a scientifically based doctrine of congenital (from the English innate immunity), or natural, natural, and adaptive (from the English adaptive immunity), or adaptive, acquired immunity (from the English acquired immunity). In immunological practice, the terms “innate” and “adaptive” immunity, innate and adaptive components are often used. immune system, innate and adaptive immune response. Both types of immunity are realized through cellular and humoral factors. Terms such as “nonspecific immunity”, “nonspecific immunological reactivity” and the like are a thing of the past.
Innate and acquired immunity represents two interacting parts of one system that ensures the development of an immune response to genetically foreign substances.
Innate immunity- a hereditarily fixed system of protection of multicellular organisms from any pathogenic and non-pathogenic microorganisms, as well as endogenous products of tissue destruction.
Like the earliest form of the body's immune defense, innate immunity was formed on initial stages the evolution of multicellular organisms, until the appearance of the ability to rearrange immunoglobulin and TCR genes, as well as the possibility of recognizing “one’s own” and full-fledged immune memory. Proof of this is the presence of various innate defense genes in invertebrate animals and plants. It is known that invertebrates (for example, arthropods) have cellular elements, which have a phagocytic function, and humoral factors such as antimicrobial peptides, lectins, etc., which successfully recognize and attack pathogenic microorganisms. All these components are conservative, inherited and are not subject to genetic modification during life.
The main distinctive features are characterized signs systems innate immunity.
Innate immunity ensures the recognition and elimination of pathogens in the first few minutes or hours after their entry into the body, when the mechanisms of adaptive immunity are still absent.
Function of the innate immune system carried out through a variety of cellular elements (macrophages, DCs, neutrophils, mast cells, eosinophils, basophils, NK cells, NKT cells, some non-hematopoietic cells) and humoral factors (natural antibodies, cytokines, complement, acute phase proteins, cationic antimicrobial peptides, lysozyme, etc.).
Cells of the innate immune system:
do not form clones. The lack of clonality in the organization of the innate immune system is one of its main differences from the adaptive immune system. In this sense, each cell of innate immunity acts individually, whereas with an adaptive immune response, all cells within a clone (community) are subject to a single genetically determined program;
are not subject to negative and positive selection;
participate in the reactions of phagocytosis, cytolysis, including bacteriolysis, neutralization, production of cytokines, etc.
Recognition pathogens by cells of the innate immune system is realized through numerous receptor structures, such as scavenger receptors (scavenger receptors), mannose receptors, complement receptors (CRl, CR3, CR4), lectin receptors, etc. Special group Innate immune receptors are so-called pattern-recognition receptors (PRR).
They recognize conservative, structures common to many types of microorganisms, the so-called Pathogen-Associated Molecular Patterns (PAMP). Currently, the structure and functions of innate immune receptors, such as Toll-like receptors (TLRs), NOD-1, NOD-2, RIG, etc., are being intensively studied. Receptors of the innate immune system are evolutionarily conserved.
Toll receptors first discovered in Drosophila. Toll-like receptors (TLRs) in mammals have a similar structure and function. Receptors of this family are widely represented on different cells immune system (monocytes, DC, leukocytes, etc.), as well as on many cells of the body (fibroblasts, endothelium, epithelium, cardiomyocytes, etc.). The TLR system is discussed in more detail below.
Factors of innate immunity do not change during the life of the organism, are controlled by germline genes and are inherited.
Activation of innate immunity does not form long-term immune memory, but serves prerequisite development of the adaptive immune response.
All of these functions are extremely important for protection against pathogenic microorganisms, but are insufficient for the life of highly organized multicellular organisms, such as vertebrates. It was in them that, in the process of evolution, new immune components arose and an immune system was formed, the main function of which was control over the genetic constancy of the internal environment of a multicellular organism. The immune system was faced with the task of recognizing and remembering “its own.” Everything that is antigenically “self” must be preserved, and everything that is antigenically “foreign” must be removed from the body. In the context of the multimillion-dollar diversity of foreign antigenic structures, it is impossible to get by with a small set of genes transmitted by inheritance (the so-called germline genes).
The general human immune system is divided into two large subsystems - nonspecific natural innate immunity and acquired specific (adaptive) immunity. Let's figure out what it is innate immunity how it works and why a person needs it. At birth, the child enters an environment different from the sterile intrauterine life. Even if all the rules of asepsis and antisepsis are followed, literally from the first second of life he is subject to attack by microorganisms. However, the baby does not get sick at the moment of birth! Why is this happening? It's all about innate immunity, which can protect a newborn from the danger of infection. Innate immunity is persistent, it is inherited, which is associated with the biological properties of the body. For example, animals do not suffer from human venereal diseases, and humans do not suffer from rinderpest. The innate immune system creates a powerful barrier against the entry of bacteria, viruses, fungi, and so on into the body.
Phagocyte cells
Innate immunity provides 60% of our body's total defense. It ensures the recognition and elimination of pathogens in the first few minutes or hours after they enter the body. Innate immunity begins to form in the middle of the first trimester of pregnancy with phagocytes. Phagocytes are cells that are capable of engulfing foreign organisms. They “grow” from stem cells and undergo “training” in the spleen, thanks to which they can subsequently distinguish between themselves and others. Phagocyte cells usually circulate throughout the body in search of foreign materials, but can be called to a specific location by cytokines. Phagocytosis is an important feature of the cellular component of innate immunity and, in all likelihood, represents the most old uniform protection of the body, since phagocytes are found in both vertebrates and invertebrates.
Factors of innate immunity
Innate immunity- This is the innate ability to destroy everything alien to the body. It is the first line of defense of the mammalian body against tumors and infectious diseases. The main external protective barrier that prevents the penetration of microorganisms into the human body is the skin and mucous membranes. The protective properties of the skin are, first of all, its impermeability ( physical barrier) and the presence of microorganism inhibitors on the surface (lactic acid and fatty acids in sweat and secretions sebaceous glands, low surface pH). The mucous membrane has a multicomponent defense mechanism. The mucus secreted by its cells prevents microorganisms from attaching to it, and the movement of the cilia helps to “sweep out” foreign substances from the respiratory tract. Tears, saliva and urine actively wash away foreign substances from the mucous membranes. Many fluids secreted by the body have specific bactericidal properties. For example, hydrochloric acid stomach, spermine and zinc in semen, lactoperoxidase in breast milk and lysozyme in many external secretions (nasal, tears, bile, duodenal contents, breast milk etc.) have powerful bactericidal properties. Some enzymes also have a bactericidal effect, for example, hyaluronidase, α1-antitrypsin, lipoproteinase.
Innate immune cells
Innate immune cells do not form clones. Each cell of the innate immune system acts individually. Factors of innate immunity do not change during the life of the organism, are controlled by germline genes and are inherited. Innate immune cells, NK cells or natural killer cells, are capable of killing a wide range of cells - from virus-infected to tumor cells. A decrease in NK cell activity and a decrease in the number of cells in the NK cell population are associated with the development and rapid progression of diseases such as cancer, viral hepatitis, AIDS, chronic fatigue syndrome, immunodeficiency syndrome and a number of autoimmune diseases. A number of studies have shown that low levels NK cell population correlates with faster spread of tumors, with a shorter life span of patients and greater mortality. An increase in the functional activity of natural killer cells is directly related to the manifestation of antiviral and antitumor effects. The search for drugs that increase the activity of this part of the innate immune system seems strategically promising for development. antiviral drugs wide spectrum of action. Therefore, leading scientists all over the world are busy searching for such drugs. Meanwhile, such a drug already exists, but more on that below.
Stimulators of innate immune cells
Unfortunately, in almost half of the population of our planet, the level of NK cells is significantly lower than normal. This condition is called immunodeficiency. Immunodeficiency leads to more frequent infectious diseases and is the cause of higher cancer morbidity and mortality. Therefore, today there is an urgent need for means that stimulate an increase in the number and functional activity of NK and, thus, leading to the elimination of immunodeficiency and helping to strengthen the immune system.  For these purposes, immunomodulators and adaptogens are used in practice. However, their activity is not high enough. It has recently been established that the most active stimulators of NK cell function are the so-called transfer factor proteins, which are contained in blood leukocytes and, as it turns out, in the colostrum of cows, goats and egg yolk. These proteins have 4-5 times higher immunostimulating activity in comparison with known active immunomodulators used in practical medicine. And due to the availability of the source of transfer factors, the possibility of obtaining it in unlimited quantities opens up. The 4Life company, inspired by the possibilities of transfer factors, began to be the first to produce the drug Transfer Factor, which is based on transfer factors from cow colostrum and egg yolks. Absorbed into the blood, transfer factors rush to the DNA molecule, check it for damage, and restore its integrity thanks to the information contained in them. The result is the debugging of all immune processes. In other words, after taking Transfer Factor, the immune system itself begins to understand when, how and how to react to this or that type of danger. Today you can buy Transfer Factor in Ukraine, to do this you just need to call us or fill out a special order form.
For these purposes, immunomodulators and adaptogens are used in practice. However, their activity is not high enough. It has recently been established that the most active stimulators of NK cell function are the so-called transfer factor proteins, which are contained in blood leukocytes and, as it turns out, in the colostrum of cows, goats and egg yolk. These proteins have 4-5 times higher immunostimulating activity in comparison with known active immunomodulators used in practical medicine. And due to the availability of the source of transfer factors, the possibility of obtaining it in unlimited quantities opens up. The 4Life company, inspired by the possibilities of transfer factors, began to be the first to produce the drug Transfer Factor, which is based on transfer factors from cow colostrum and egg yolks. Absorbed into the blood, transfer factors rush to the DNA molecule, check it for damage, and restore its integrity thanks to the information contained in them. The result is the debugging of all immune processes. In other words, after taking Transfer Factor, the immune system itself begins to understand when, how and how to react to this or that type of danger. Today you can buy Transfer Factor in Ukraine, to do this you just need to call us or fill out a special order form.
Strengthening immunity. However, few of us know that the concept of the immune system has its own types and characteristics. What is human immunity? Let's figure it out together.
Some terminology
This concept hides the body’s ability to prevent the activity of bacteria, toxins and other harmful substances. Doctors distinguish between such types of immunity as innate and acquired, which, in turn, are divided into other forms, which we will talk about a little later.
The main task of the body's immune system is to maintain the health and normal functioning of all human organs and systems. Thus, immunity plays the role of a protective barrier that separates us from environment. Let's take a closer look at the types and forms of protective functions of the human body.
Innate immunity
This type of protection is associated with characteristics of the body that are inherited at birth. The functioning of innate immunity is ensured by many cellular and non-cellular (so-called) factors. For example, the skin and mucous membranes are a reliable barrier for most microbes. The body is also protected by sweat, sebaceous, salivary glands. The substances they release are harmful to most pathogenic bacteria. Normal intestinal microflora contains microorganisms that are natural enemies of many pathogens. They also fight infection in the digestive tract gastric juice, enzymes and bile.
The body's natural defenses are very strong. But its enemies - microorganisms, foreign molecules and cells - are constantly trying to penetrate inside, disrupting the integrity of barriers or the normal secretion of organs and systems - lowering natural immunity. Provoking factors in such a situation are hypothermia or stress, lack of vitamins or medication, hormonal imbalance or surgical intervention. In this case, the penetration of microorganisms into the body is greatly facilitated. But here another type of body defense comes into force. We'll talk about it in detail later.
Acquired immunity
If a foreign agent has penetrated the natural barrier into the body’s bloodstream, then several options for the relationship between the immune system and bacteria may arise, one of which is an infectious disease. In this case, acquired immunity is activated, which will fight the infection in the future.
The main characteristic of this type of immunity is the production of specific antibodies against a particular antigen. It is formed during a person’s life and is not inherited. The uniqueness of acquired immunity lies in the fact that, depending on the diseases suffered, it changes, producing new antibodies. This type of protective functions of the body can be natural or artificially acquired. Let us consider these forms of immunity in detail.
If a protective immune barrier appears after past illness, it is called natural acquired immunity. After an attack by pathogens, the body itself produces antibodies. Sometimes they protect the body from re-infection for weeks and months (with influenza, ARVI), and maybe even for long years or throughout life, as with measles or scarlet fever (this immunity is called persistent).
When a person is injected with weakened pathogens that cause an immune response in the body, we are talking about artificially acquired active immunity. If ready-made antibodies are introduced into the body, passive immunity occurs, which allows you to protect a person who has had contact with the patient in the shortest possible time. But this form of the body’s protective functions is weaker compared to the active type of acquired immune barrier.
A striking representative of passive immunity is the newborn child. While still in the womb, the baby receives antibodies through the placenta against the pathogens of the diseases that the mother suffered from. By 3-6 months of life, this type of immunity weakens, and by the end of the first year of life it completely fades away. But it can be strengthened by practicing breastfeeding.
Summarizing all of the above, we can conclude that immunity is a rather complex system that requires constant outside help in the form of vaccination, compliance with hygiene rules, healthy eating And physical activity. That is, to be healthy, you just need to follow these rules.
9.1. Introduction to Immunology9.1.1. Main stages in the development of immunology
Every person on the planet (except for identical twins) has unique genetically determined characteristics of the biopolymers from which his body is built. However, his body lives and develops in direct contact with representatives of living and inanimate nature and various bioorganic molecules of natural or artificial origin that have biological activity. When entering the human body, waste products and tissues of other people, animals, plants, microbes, as well as foreign molecules can interfere and disrupt biological processes, posing a threat to the life of an individual. Distinctive feature of these agents is genetic foreignness. Often, such products are formed inside the human body as a result of the synthetic activity of the microflora inhabiting us, cellular mutations and various modifications of the macromolecules from which we are built.
To protect against unwanted and destructive intervention, evolution has created in representatives of living nature special system counteraction, the cumulative effect of which was designated as immunity(from lat. immunitas- liberation from something, inviolability). This term was used already in the Middle Ages to designate, for example, exemption from paying taxes, and later - the inviolability of a diplomatic mission. The meaning of this term exactly corresponds to the biological tasks that evolution has determined in relation to immunity.
The main ones are the recognition of the genetic difference between the interventionist and one’s own structures and the elimination of its influence on the biological processes occurring in the body using a set of special reactions and mechanisms. The ultimate goal of the immune defense system is the preservation of homeostasis, structural and functional integrity and genetic individuality of both an individual organism and the species as a whole, as well as the development of means of preventing such interventions in the future.
Consequently, immunity is a way of protecting the body from genetically foreign substances of exogenous and endogenous origin, aimed at maintaining and preserving homeostasis, the structural and functional integrity of the body and the genetic individuality of each organism and species as a whole.
Immunity as a general biological and general medical phenomenon, its anatomical structures, and mechanisms of functioning in the body are studied by a special science - immunology. This science originated more than 100 years ago. As human knowledge progressed, views on immunity, its role in the body, and the mechanisms of immune reactions changed, the scope of practical use of the achievements of immunology expanded, and in accordance with this, the very definition of immunology as a science changed. Immunology is often interpreted as a science that studies specific immunity to pathogens of infectious diseases and develops methods of protection against them. This is a one-sided view that does not provide a comprehensive, comprehensive understanding of science, based on the essence and mechanisms of immunity and its role in the life of the body. On modern stage development of the doctrine of immunity, immunology can be defined as a general biological and general medical science that studies the methods and mechanisms of protecting the body from genetically foreign substances of exogenous and endogenous origin in order to maintain homeostasis, the structural and functional integrity of the body and the genetic individuality of an individual and the species as a whole. This definition emphasizes that immunology as a science is unified regardless of the object of study: humans, animals or plants. Of course, the anatomical and physiological basis, a set of mechanisms and reactions, as well as methods of protection against antigens in animal representatives
And flora will vary, but the fundamental essence of immunity will not change. In immunology, there are three areas: medical immunology (homoimmunology), zooimmunology and phytoimmunology, which study immunity in humans, animals and plants, respectively, and in each of them - general and specific. One of its most important sections is medical immunology. Today, medical immunology solves such important problems as the diagnosis, prevention and treatment of infectious diseases (immunoprevention or vaccinology), allergic conditions (allergology), malignant tumors(immuno-oncology), diseases in the mechanism of which immunopathological processes play a role (immunopathology), immune relationships between mother and fetus at all stages of reproduction (immunology of reproduction), studies immune mechanisms and makes a practical contribution to solving the problem of organ and tissue transplantation (transplantation immunology); One can also distinguish immunohematology, which studies the relationship between donor and recipient during blood transfusion, immunopharmacology, which studies the effect on immune processes medicinal substances. IN last years clinical and environmental immunology were distinguished. Clinical immunology studies and develops the problems of diagnosis and treatment of diseases arising as a result of congenital (primary) and acquired (secondary) immunodeficiencies, and environmental immunology is the influence on the immune system of all kinds environmental factors(climate-geographical, social, professional, etc.).
Chronologically, immunology as a science has already gone through two large periods (Ulyankina T.I., 1994): the period of protoimmunology (from ancient period until the 80s of the 19th century), associated with spontaneous, empirical knowledge of the body’s defense reactions, and the period of the emergence of experimental and theoretical immunology (from the 80s of the 19th century to the second decade of the 20th century). During the second period, the formation of classical immunology, which was mainly in the nature of infectious immunology, was completed. Since the middle of the 20th century, immunology has entered the third, molecular genetic, period, which continues to this day. This period is characterized by rapid development of molecular and cellular immunology and immunogenetics.
Prevention from smallpox by inoculating humans with cowpox was proposed more than 200 years ago English doctor E. Jenner, however, this observation was purely empirical. Therefore, the French chemist L. Pasteur, who discovered the principle of vaccination, and the Russian zoologist I.I. are rightfully considered the founders of scientific immunology. Mechnikov is the author of the doctrine of phagocytosis and the German biochemist P. Ehrlich, who formulated the hypothesis of antibodies. In 1888, for the outstanding services of L. Pasteur to humanity, the Institute of Immunology (now the Pasteur Institute) was established with public donations, which was a school around which immunologists from many countries were grouped. Russian scientists actively participated in the formation and development of immunology. For more than 25 years I.I. Mechnikov was deputy director for science at the Pasteur Institute, i.e. was his closest assistant and like-minded person. Many outstanding Russian scientists worked at the Pasteur Institute: M. Bezredka, N.F. Gamaleya, L.A. Tarasovich, G.N. Gabrichevsky, I.G. Savchenko, S.V. Korshun, D.K. Zabolotny, V.A. Barykin, N.Ya. and F.Ya. Chistovichi and many others. These scientists continued to develop the traditions of Pasteur and Mechnikov in immunology and essentially created the Russian school of immunologists.
Russian scientists own many outstanding discoveries in the field of immunology: I.I. Mechnikov laid the foundations of the doctrine of phagocytosis, V.K. Vysokovych was one of the first to formulate the role of the reticuloendothelial system in immunity, G.N. Gabrichevsky described the phenomenon of leukocyte chemotaxis, F.Ya. Chistovich stood at the origins of the discovery of tissue antigens, M. Raisky established the phenomenon of revaccination, i.e. immunological memory, M. Sakharov - one of the founders of the doctrine of anaphylaxis, academician. L.A. Zilber stood at the origins of the doctrine of tumor antigens, academician. P.F. Zdrodovsky substantiated the physiological direction in immunology, academician. R.V. Petrov made a significant contribution to the development of non-infectious immunology.
Russian scientists are rightfully leaders in the development of fundamental and applied problems of vaccinology and immunoprophylaxis in general. The names of the creators of vaccines against tularemia (B.Ya. Elbert and N.A. Gaisky) are well known in our country and abroad. anthrax(N.N. Ginzburg), polio-
lita (M.P. Chumakov, A.A. Smorodintsev), measles, mumps, influenza (A.A. Smorodintsev), Q fever and typhus (P.F. Zdrodovsky), polyanatoxins against wound infections and botulism (A A. Vorobyov, G. V. Vygodchikov, P. N. Burgasov) and others. Russian scientists took an active part in the development of vaccines and other immunobiological drugs, strategies and tactics of immunoprophylaxis, global elimination and reduction of infectious diseases. In particular, on their initiative and with their help, smallpox was eradicated from the globe (V.M. Zhdanov, O.G. Andzhaparidze), polio was successfully eradicated (M.P. Chumakov, S.G. Drozdov).
Over a relatively short historical period, immunology has achieved significant results in reducing and eliminating human diseases, preserving and maintaining the health of the people of our planet.
9.1.2. Types of immunity
The ability to recognize foreign structures and protect one’s own body from invaders was formed quite early. Lower organisms, in particular invertebrates (sponges, coelenterates, worms), already have elementary systems of protection against any foreign substances. The human body, like all warm-blooded animals, already has a complex system of counteracting genetically foreign agents. However, the anatomical structure, physiological functions and reactions that provide such protection in certain animal species, in humans and lower organisms in accordance with the level of evolutionary development differ significantly.
Thus, phagocytosis and allogeneic inhibition, as one of the early phylogenetic defense reactions, is inherent in all multicellular organisms; differentiated leukocyte-like cells that perform the functions of cellular immunity already appear in coelenterates and mollusks; in cyclostomes (lamreys) thymus rudiments, T-lymphocytes, immunoglobulins appear, and immune memory is noted; fish already have lymphoid organs typical of higher animals - the thymus and spleen, plasma cells and class M antibodies; birds have central authority immunity in the form of a bursa of Fabricius, they have the ability to react in the form of hypersensitivity immediately
new type. Finally, in mammals the immune system reaches its most high level development: T-, B- and A-systems are formed immune cells, their cooperative interaction occurs, the ability to synthesize immunoglobulins of different classes and forms of immune response appears.
Depending on the level of evolutionary development, the characteristics and complexity of the formed immune system, and the ability of the latter to respond with certain reactions to antigens, in immunology it is customary to distinguish separate types of immunity.
Thus, the concept of innate and acquired immunity was introduced (Fig. 9.1). Innate, or species, immunity, also known as hereditary, genetic, constitutional, is the genetically fixed, inherited immunity of individuals of a given species to any foreign agent developed in the process of phylogenesis. An example is human immunity to certain pathogens, including those that are especially dangerous for farm animals (rinderpest, Newcastle disease, which affects birds, horse pox, etc.), and human insensitivity to bacteriophages that infect bacterial cells. Species immunity can be explained from different positions: the inability of a foreign agent to adhere to cells and target molecules that determine the initiation of the pathological process and activation of the immune system, its rapid destruction by enzymes of the macroorganism, and the absence of conditions for colonization of the macroorganism.
Species immunity may be absolute And relative. For example, frogs that are insensitive to tetanus toxin respond to its administration when their body temperature rises. Laboratory animals that are insensitive to any foreign agent react to it against the background of the introduction of immunosuppressants or the removal of the central organ of immunity - the thymus.
Acquired immunity is immunity to a foreign agent in a human or animal body that is sensitive to it, acquired in the process of individual development, i.e. development of each individual individually. Its basis is the potential for immune protection, which is realized only when necessary and under certain conditions. Acquired immunity, or rather its final result, is not inherited in itself (unlike, of course, potency); it is an individual lifetime experience.
Rice. 9.1. Classification of types of immunity
Distinguish natural And artificial acquired immunity. An example of natural acquired immunity in humans is immunity to infection that occurs after a history of infectious disease(so-called post-infectious immunity), for example after scarlet fever. Artificial acquired immunity is created deliberately to create immunity in the body
to a specific agent by introducing special immunobiological preparations, for example vaccines, immune sera, immunocompetent cells (see Chapter 14).
Acquired immunity can be active And passive. Active immunity due to the direct involvement of the immune system in the process of its formation (for example, post-vaccination, post-infectious immunity). Passive immunity is formed by introducing ready-made immunoreagents into the body that can provide the necessary protection. These drugs include antibodies (immunoglobulin preparations and immune serums) and lymphocytes. Passive immunity is formed in the fetus in the embryonic period due to the penetration of maternal antibodies through the placenta, and during breastfeeding - when the child absorbs antibodies contained in milk.
Since cells of the immune system and humoral factors take part in the formation of immunity, it is accepted active immunity differentiate depending on which component of the immune response plays the leading role in the formation of protection against the antigen. In this regard, there is a distinction humoral, cellular immunity. An example of cellular immunity is transplantation immunity, when the leading role in immunity is played by cytotoxic killer T-lymphocytes. Immunity during toxinemic infections (diphtheria) and intoxications (tetanus, botulism) is mainly due to antibodies (antitoxins).
Depending on the direction of immunity, i.e. nature of the foreign agent, emit antitoxic, antiviral, antifungal, antibacterial, antiprotozoal, transplantation, antitumor and other types of immunity.
Immunity can be maintained or maintained either in the absence or only in the presence of a foreign agent in the body. In the first case, such an agent plays the role of a triggering factor, and immunity is called sterile, in the second - non-sterile. An example of sterile immunity is post-vaccination immunity with the introduction of killed vaccines, and non-sterile immunity is immunity in tuberculosis, which is maintained by the constant presence of Mycobacterium tuberculosis in the body.
Immunity may be systemic those. generalized, spreading throughout the entire body, and local, at which
More pronounced resistance of individual organs and tissues is observed. As a rule, taking into account the features anatomical structure and organization of functioning, the concept of “local immunity” is used to refer to the resistance of the mucous membranes (therefore it is sometimes called mucosal) and skin. This division is also conditional, since in the process of developing immunity these types of immunity can transform into each other.
9.2. Innate immunity
Congenital(species, genetic, constitutional, natural, nonspecific) immunity- this is resistance to infectious agents (or antigens) developed in the process of phylogenesis, inherited, and inherent in all individuals of the same species.
The main feature of the biological factors and mechanisms that ensure such resistance is the presence in the body of ready-made (preformed) effectors that are capable of ensuring the destruction of the pathogen quickly, without lengthy preparatory reactions. They constitute the body's first line of defense against external microbial or antigenic aggression.
9.2.1. Factors of innate immunity
If we consider the trajectory of a pathogenic microbe in the dynamics of the infectious process, it is easy to notice that the body builds various lines of defense along this path (Table 9.1). First of all, it is the integumentary epithelium of the skin and mucous membranes, which has colonization resistance. If the pathogen is armed with appropriate invasive factors, then it penetrates the subepithelial tissue, where an acute inflammatory reaction develops, limiting the pathogen at the entrance gate. The next station on the path of the pathogen is the regional lymph nodes, where it is transported by lymph through lymphatic vessels, draining connective tissue. Lymphatic vessels and nodes respond to penetration by developing lymphangitis and lymphadenitis. After overcoming this barrier, microbes penetrate into the blood through the efferent lymphatic vessels - in response, a systemic inflammatory response can develop.
vet. If the microbe does not die in the blood, then it spreads hematogenously to the internal organs - generalized forms of infection develop.
Table 9.1. Factors and mechanisms of anti-infectious immunity (the principle of echeloning of antimicrobial defense according to Mayansky A.N., 2003)
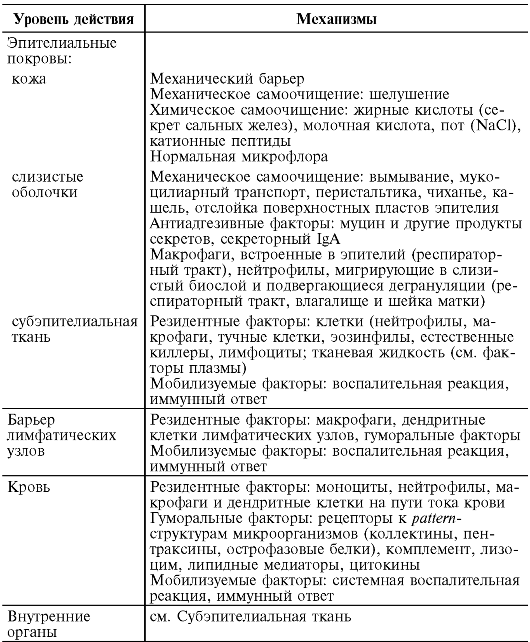 Factors of innate immunity include:
Factors of innate immunity include:
Skin and mucous membranes;
Cellular factors: neutrophils, macrophages, dendritic cells, eosinophils, basophils, natural killer cells;
Humoral factors: complement system, soluble receptors for the surface structures of microorganisms (pattern structures), antimicrobial peptides, interferons.
Skin and mucous membranes. The thin layer of epithelial cells lining the surface of the skin and mucous membranes is a barrier that is practically impenetrable to microorganisms. It separates the body's sterile tissues from the microbial outside world.
Leather covered with multilayered squamous epithelium, in which two layers are distinguished: horny and basal.
Keratinocytes of the stratum corneum are dead cells that are resistant to aggressive chemical compounds. There are no receptors on their surface for adhesive molecules of microorganisms, therefore they have significant resistance to colonization and are the most reliable barrier to most bacteria, fungi, viruses, and protozoa. The exception is S. aureus, Pr. acnae, I. pestis, and they most likely penetrate either through microcracks, or with the help of blood-sucking insects, or through the mouths of the sweat and sebaceous glands. The mouth of the sebaceous and sweat glands, hair follicles in the skin are the most vulnerable, since here the layer of keratinized epithelium becomes thinner. In protecting these areas, products of the sweat and sebaceous glands, containing lactic and fatty acids, enzymes, and antibacterial peptides that have an antimicrobial effect, play an important role. It is in the mouths of the skin appendages that deep resident microflora is located, forming microcolonies and producing protective factors (see Chapter 4).
In addition to keratinocytes, the epidermis contains two more types of cells - Langerhans cells and Greenstein cells (processed epidermocytes, constituting 1-3% of the karyocytes of the basal layer). Langerhans and Greenstein cells are of myeloid origin and belong to dendritic cells. It is assumed that these cells are opposite in function. Langerhans cells are involved in antigen presentation and induce an immune response, and Greenstein cells produce cytokines that suppress the immune response.
mune reactions in the skin. Typical keratinocytes and dendritic cells of the epidermis, together with the lymphoid structures of the dermis, take an active part in the reactions of acquired immunity (see below).
Healthy skin has high ability to self-purification. This is easy to prove if you apply bacteria atypical for skin to its surface - after a while such microbes disappear. Methods for assessing the bactericidal function of the skin are based on this principle.
Mucous membranes. Most infections begin not from the skin, but from the mucous membranes. This is due, firstly, to larger area their surfaces (mucous membranes about 400 m 2, skin about 2 m 2), secondly, with less protection.
The mucous membranes do not have stratified squamous epithelium. On their surface there is only one layer of epithelial cells. In the intestine, these are single-layer columnar epithelium, goblet secretory cells and M-cells (membrane epithelial cells), located in the layer of epithelial cells, covering lymphoid accumulations. M cells are the most vulnerable to the penetration of many pathogenic microorganisms due to a number of features: the presence of specific receptors for some microorganisms (Salmonella, Shigella, pathogenic Escherichia, etc.), which are not found on neighboring enterocytes; thinned mucous layer; the ability to endocytosis and pipocytosis, which ensures facilitated transport of antigens and microorganisms from the intestinal tube into mucous-associated lymphoid tissue (see Chapter 12); the absence of a powerful lysosomal apparatus, characteristic of macrophages and neutrophils, due to which bacteria and viruses move into the subepithelial space without destruction.
M cells belong to an evolutionarily formed system of facilitated transport of antigens to immunocompetent cells, and bacteria and viruses use this pathway for their translocation through the epithelial barrier.
Epithelial cells, similar to intestinal M-cells, associated with lymphoid tissue, are present in the mucous membranes of the bronchoalveolar tree, nasopharynx, and reproductive system.
Colonization resistance of the integumentary epithelium. Any infectious process begins with adhesion of the pathogen to the
the surface of sensitive epithelial cells (with the exception of microorganisms transmitted through insect bites or vertically, i.e. from mother to fetus). Only after gaining a foothold do microbes acquire the ability to multiply at the entrance gate and form a colony. Toxins and pathogenicity enzymes accumulate in the colony in quantities necessary to overcome the epithelial barrier. This process is called colonization. Colonization resistance is understood as the resistance of the epithelium of the skin and mucous membranes to colonization by foreign microorganisms. Colonization resistance of mucous membranes is provided by mucin, secreted by goblet cells and forming a complex biofilm on the surface. All protective tools are built into this biolayer: resident microflora, bactericidal substances (lysozyme, lactoferrin, toxic metabolites of oxygen, nitrogen, etc.), secretory immunoglobulins, phagocytes.
The role of normal microflora(see chapter 4.3). The most important mechanism for the participation of resident microflora in colonization resistance is their ability to produce bacteriocins (antibiotic-like substances), short-chain fatty acids, lactic acid, hydrogen sulfide, and hydrogen peroxide. Lacto-, bifidobacteria, and bacteroides have these properties.
Thanks to the enzymatic activity of anaerobic bacteria in the intestine, bile acids are deconjugated to form deoxycholic acid, which is toxic to pathogenic and opportunistic bacteria.
Mucin along with polysaccharides produced by resident bacteria (in particular, lactobacilli), it forms a pronounced glyconalix (biofilm) on the surface of the mucous membranes, which effectively screens adhesion sites and makes them inaccessible to random bacteria. Goblet cells form a mixture of sialo- and sulfomycins, the ratio of which varies in different biotones. The unique composition of microflora in various ecological niches is largely determined by the quantity and quality of mucin.
Phagocytic cells and their degranulation products. Macrophages and neutrophils migrate into the mucous biolayer on the surface of the epithelium. Along with phagocytosis, these cells secrete biocide
outward products contained in their lysosomes (lysozyme, peroxidase, lactoferrin, defansins, toxic oxygen and nitrogen metabolites), which increase the antimicrobial properties of the secretions.
Chemical and mechanical factors. In the resistance of the integumentary epithelium of the mucous membranes, an important role is played by secretions that have pronounced biocidal and anti-adhesive properties: tears, saliva, gastric juice, enzymes and bile acids of the small intestine, cervical and vaginal secretions of the female reproductive system.
Thanks to targeted movements - peristalsis of smooth muscles in the intestines, cilia of the ciliated epithelium in the respiratory tract, urine in urinary system- the resulting secretions, together with the microorganisms contained in them, move in the direction of the exit and are brought out.
Colonization resistance of mucous membranes is enhanced by secretory immunoglobulins A, synthesized by mucous-associated lymphoid tissue.
The integumentary epithelium of the mucosal tract constantly regenerates due to stem cells located in the thickness of the mucous membranes. In the intestine, this function is performed by crypt cells, in which, along with stem cells, Paneth cells are located - special cells that synthesize antibacterial proteins (lysozyme, cationic peptides). These proteins protect not only stem cells, but also integumentary epithelial cells. With inflammation in the wall of the mucous membrane, the production of these proteins increases.
Colonization resistance of the integumentary epithelium is ensured by the entire set of protective mechanisms of innate and acquired (secretory immunoglobulins) immunity and is the basis of the body’s resistance to most microorganisms that live in external environment. The absence of specific receptors for certain microorganisms on epithelial cells appears to be the basic mechanism of genetic resistance of animals of one species to microbes that are pathogenic for animals of another species.
9.2.2. Cellular factors
Neutrophils and macrophages. The ability for endocytosis (the absorption of particles with the formation of an intracellular vacuole) is
produced by all eukaryotic cells. This is how many pathogenic microorganisms penetrate into cells. However, in most infected cells there are no mechanisms (or they are weak) that ensure the destruction of the pathogen. In the process of evolution, specialized cells with powerful intracellular killing systems were formed in the body of multicellular organisms, the main “profession” of which is phagocytosis (from the Greek. phagos- I devour, cytos- cell) - absorption of particles with a diameter of at least 0.1 microns (as opposed to pinocytosis - absorption of particles of smaller diameter and macromolecules) and destruction of captured microbes. Polymorphonuclear leukocytes (mainly neutrophils) and mononuclear phagocytes (these cells are sometimes called professional phagocytes) have these properties.
For the first time the idea of protective role motile cells (micro- and macrophages) was formulated in 1883 by I.I. Mechnikov, who was awarded the Nobel Prize in 1909 for the creation of the cellular-humoral theory of immunity (in collaboration with P. Ehrlich).
Neutrophils and mononuclear phagocytes have a common myeloid origin from the hematopoietic stem cell. However, these cells differ in a number of properties.
Neutrophils are the most numerous and mobile population of phagocytes, the maturation of which begins and ends in the bone marrow. About 70% of all neutrophils are stored as a reserve in the bone marrow depots, from where, under the influence of appropriate stimuli (proinflammatory cytokines, products of microbial origin, the C5a component of complement, colony-stimulating factors, corticosteroids, catecholamines) they can urgently move through the blood to the site of tissue destruction and participate in the development of acute inflammatory response. Neutrophils are the “rapid response team” in the antimicrobial defense system.
Neutrophils are short-lived cells, their lifespan is about 15 days. From the bone marrow they enter the bloodstream as mature cells that have lost the ability to differentiate and proliferate. From the blood, neutrophils move to tissues, where they either die or come to the surface of the mucous membranes, where they complete their life cycle.
Mononuclear phagocytes are represented by bone marrow promonocytes, blood monocytes and tissue macrophages. Monocytes, unlike neutrophils, are immature cells that, when entering bloodstream and further in the tissue, mature into tissue macrophages (pleural and peritoneal, Kupffer cells of the liver, alveolar, interdigital cells of the lymph nodes, bone marrow, osteoclasts, microgliocytes, mesangial cells of the kidneys, Sertoli cells of the testicles, Langerhans and Greenstein cells of the skin). The lifespan of mononuclear phagocytes is from 40 to 60 days. Macrophages are not very fast cells, but they are scattered throughout all tissues, and, unlike neutrophils, they do not need such urgent mobilization. If we continue the analogy with neutrophils, then macrophages in the innate immune system are “special forces”.
An important feature of neutrophils and macrophages is the presence in their cytoplasm of a large number of lysosomes - granules 200-500 nm in size containing various enzymes, bactericidal and biologically active products (lysozyme, myeloperoxidase, defensins, bactericidal protein, lactoferrin, proteinases, cathepsins, collagenase, etc.). d.). Thanks to such diverse “weapons,” phagocytes have powerful destructive and regulatory potential.
Neutrophils and macrophages are sensitive to any changes in homeostasis. For this purpose, they are equipped with a rich arsenal of receptors located on their cytoplasmic membrane (Fig. 9.2):
Receptors for foreign recognition - Toll-like receptors (Toll-like receptor- TLR), first discovered by A. Poltorak in 1998 in the fruit fly and subsequently found in neutrophils, macrophages and dendritic cells. The significance of the discovery of Toll-like receptors is comparable to the earlier discovery of antigen recognition receptors in lymphocytes. Toll-like receptors recognize not antigens, the diversity of which in nature is extremely large (about 10 18 variants), but coarser repeating molecular carbohydrate and lipid patterns - pattern structures (from the English. pattern- pattern), which are not on the cells of the host body, but which are present in protozoa, fungi, bacteria, viruses. The repertoire of such patterns is small and amounts to about 20
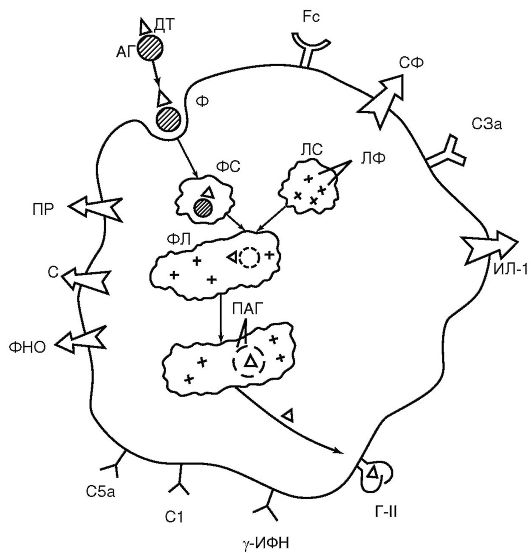 Rice. 9.2. Functional structures of a macrophage (diagram): AG - antigen; DT - antigenic determinant; FS - phagosome; LS - lysosome; LF - lysosomal enzymes; PL - phagolysosome; PAG - processed antigen; G-II - class II histocompatibility antigen (MHC II); Fc - receptor for the Fc fragment of the immunoglobulin molecule; C1, C3a, C5a - receptors for complement components; γ-IFN - receptor for γ-MFN; C - secretion of complement components; PR - secretion of peroxide radicals; ILD-1 - secretion; TNF - secretion of tumor necrosis factor; SF - secretion of enzymes
Rice. 9.2. Functional structures of a macrophage (diagram): AG - antigen; DT - antigenic determinant; FS - phagosome; LS - lysosome; LF - lysosomal enzymes; PL - phagolysosome; PAG - processed antigen; G-II - class II histocompatibility antigen (MHC II); Fc - receptor for the Fc fragment of the immunoglobulin molecule; C1, C3a, C5a - receptors for complement components; γ-IFN - receptor for γ-MFN; C - secretion of complement components; PR - secretion of peroxide radicals; ILD-1 - secretion; TNF - secretion of tumor necrosis factor; SF - secretion of enzymes
riants. Toll-like receptors are a family of membrane glycoproteins; 11 types of such receptors are known, capable of recognizing the entire palette pattern-structures of microorganisms (lipopolysaccharides, glyco-, lipoproteins-
ys, nucleic acids, proteins heat shock etc.). The interaction of Toll-like receptors with appropriate ligands triggers the transcription of genes for pro-inflammatory cytokines and co-stimulatory molecules, which are necessary for migration, cell adhesion, phagocytosis and the presentation of antigens to lymphocytes;
Mannose-fucose receptors that recognize carbohydrate components of the surface structures of microorganisms;
Receptors for garbage (scavenger receptor)- for binding phospholipid membranes and components of own destroyed cells. Participate in phagocytosis of damaged and dying cells;
Receptors for C3b and C4b complement components;
Receptors for Fc fragments of IgG. These receptors, like receptors for complement components, play an important role in the binding of immune complexes and phagocytosis of bacteria labeled with immunoglobulins and complement (opsonization effect);
Receptors for cytokines, chemokines, hormones, leukotrienes, prostaglandins, etc. allow you to interact with lymphocytes and respond to any changes in the internal environment of the body.
The main function of neutrophils and macrophages is phagocytosis. Phagocytosis is the process of absorption of particles or large macromolecular complexes by a cell. It consists of several sequential stages:
Activation and chemotaxis - the targeted movement of a cell towards the object of phagocytosis towards an increasing concentration of chemoattractants, the role of which is played by chemokines, components of complement and microbial cells, products of degradation of body tissues;
Adhesion (attachment) of particles to the surface of the phagocyte. Toll-like receptors play an important role in adhesion, as well as receptors for the Fc fragment of immunoglobulin and the C3b component of complement (this phagocytosis is called immune). Immunoglobulins M, G, C3b-, C4b-complement components enhance adhesion (they are opsonins) and serve as a bridge between the microbial cell and the phagocyte;
Absorption of particles, their immersion in the cytoplasm and formation of a vacuole (phagosome);
Intracellular killing (killing) and digestion. After absorption, the phagosome particles merge with lysosomes - a phagolysosome is formed, in which the bacteria die under the influence of the bactericidal products of the granules (oxygen-independent bactericidal system). At the same time, the consumption of oxygen and glucose in the cell increases - a so-called respiratory (oxidative) explosion develops, which leads to the formation of toxic metabolites of oxygen and nitrogen (H 2 O 2, superoxide anion O 2, hypochlorous acid, pyroxynitrite), which are highly bactericidal (oxygen-dependent bactericidal system ). Not all microorganisms are sensitive to bactericidal systems of phagocytes. Gonococci, streptococci, mycobacteria and others survive after contact with phagocytes; such phagocytosis is called incomplete.
Phagocytes, in addition to phagocytosis (endocytosis), can carry out their cytotoxic reactions by exocytosis - releasing their granules outward (degranulation) - thus phagocytes carry out extracellular killing. Neutrophils, unlike macrophages, are capable of forming extracellular bactericidal traps - during the activation process, the cell throws out DNA strands in which granules with bactericidal enzymes are located. Due to the stickiness of DNA, bacteria stick to the traps and are killed by the enzyme.
Neutrophils and macrophages are the most important component of innate immunity, but their role in protection against various microbes is different. Neutrophils are effective against infections caused by extracellular pathogens (pyogenic cocci, enterobacteria, etc.) that induce the development of an acute inflammatory response. Neutrophil-complement-antibody cooperation is effective in such infections. Macrophages protect against intracellular pathogens (mycobacteria, rickettsia, chlamydia, etc.) that cause the development of chronic granulomatous inflammation, where macrophage-T-lymphocyte cooperation plays a major role.
In addition to participating in antimicrobial defense, phagocytes are involved in removing dying, old cells and their decay products, inorganic particles (coal, mineral dust, etc.) from the body. Phagocytes (especially macrophages) are antigen-preparing
constituents, they have a secretory function, synthesize and secrete out wide range biologically active compounds: cytokines (interleukins-1, 6, 8, 12, tumor necrosis factor), prostaglandins, leukotrienes, interferons α and γ. Thanks to these mediators, phagocytes actively participate in maintaining homeostasis, in the processes of inflammation, in the adaptive immune response, and regeneration.
Eosinophils belong to polymorphonuclear leukocytes. They differ from neutrophils in that they have weak phagocytic activity. Eosinophils ingest some bacteria, but their intracellular killing is less efficient than that of neutrophils.
Natural killers. Natural killer cells are large lymphocyte-like cells that arise from lymphoid precursors. They are found in the blood and tissues, especially in the liver, the mucous membrane of the female reproductive system, and the spleen. Natural killer cells, like phagocytes, contain lysosomes, but do not have phagocytic activity.
Natural killer cells recognize and eliminate target cells that have altered or absent markers characteristic of healthy cells. This is known to happen primarily to cells that have been mutated or infected by a virus. That is why natural killer cells play an important role in antitumor surveillance, the destruction of cells infected with viruses. Natural killer cells exert their cytotoxic effect with the help of a special protein, perforin, which, like the membrane-attack complement complex, forms pores in the membranes of target cells.
9.2.3. Humoral factors
Complement system. The complement system is a multicomponent multienzyme self-assembling system of serum proteins that are normally in an inactive state. When microbial products appear in the internal environment, a process called complement activation is triggered. Activation occurs as a cascade reaction, when each previous component of the system activates the next one. During the self-assembly of the system, active protein breakdown products are formed, which perform three important functions: they cause membrane perforation and cell lysis, provide opsonization of microorganisms for their further phagocytosis, and initiate the development of vascular inflammatory reactions.
The complement called “alexin” was described in 1899 by the French microbiologist J. Bordet, and then called complement by the German microbiologist P. Ehrlich (complement- addition) as a factor additional to antibodies that cause cell lysis.
The complement system includes 9 main proteins (designated as C1, C2-C9), as well as subcomponents - the breakdown products of these proteins (Clg, C3b, C3a, etc.), inhibitors.
The key event for the complement system is its activation. It can occur in three ways: classical, lectin and alternative (Fig. 9.3).
The classic way. In the classical pathway, the activating factor is antigen-antibody complexes. In this case, the Fc fragment and IgG of immune complexes are activated by the Cr subcomponent, Cr is cleaved to form Cls, which hydrolyzes C4, which is cleaved into C4a (anaphylotoxin) and C4b. C4b activates C2, which, in turn, activates the C3 component (a key component of the system). The C3 component is cleaved into anaphylotoxin C3a and opsonin C3b. Activation of the C5 component of complement is also accompanied by the formation of two active protein fragments: C5a - anaphylotoxin, a chemoattractant for neutrophils and C5b - activating the C6 component. As a result, complex C5, b, 7, 8, 9 is formed, which is called membrane attack. The terminal phase of complement activation is the formation of a transmembrane pore in the cell and the release of its contents to the outside. As a result, the cell swells and lyses.
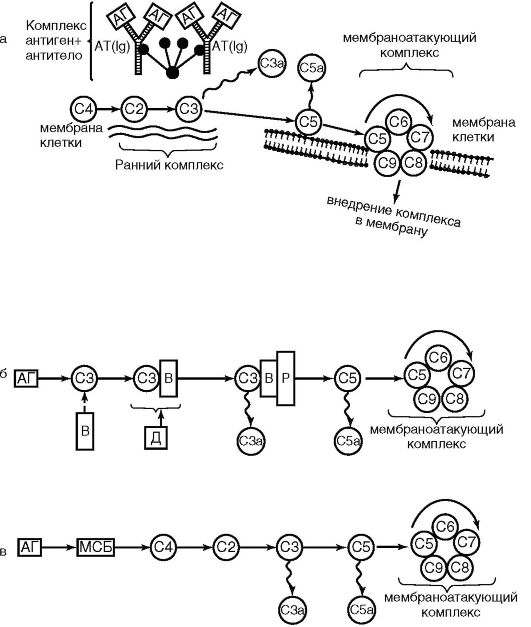 Rice. 9.3. Complement activation pathways: classical (a); alternative (b); lectin (c); C1-C9 - complement components; AG - antigen; AT - antibody; ViD - proteins; P - properdin; MBP - mannose binding protein
Rice. 9.3. Complement activation pathways: classical (a); alternative (b); lectin (c); C1-C9 - complement components; AG - antigen; AT - antibody; ViD - proteins; P - properdin; MBP - mannose binding protein
Lectin pathway. It is in many ways similar to the classic one. The only difference is that in the lectin pathway, one of the acute phase proteins, mannose-binding lectin, interacts with mannose on the surface of microbial cells (the prototype of the antigen-antibody complex), and this complex activates C4 and C2.
Alternative way. It occurs without the participation of antibodies and bypasses the first 3 components C1-C4-C2. The alternative pathway is initiated by components of the cell wall of gram-negative bacteria (lipopolysaccharides, peptidoglycans), viruses that bind sequentially to proteins P (properdin), B and D. These complexes directly convert the C3 component.
A complex cascade reaction of complement occurs only in the presence of Ca and Mg ions.
Biological effects of complement activation products:
Regardless of the path, complement activation ends with the formation of the membrane attack complex (C5, b, 7, 8, 9) and cell lysis (bacteria, erythrocytes and other cells);
The resulting C3a, C4a and C5a components are anaphylotoxins, they bind to the receptors of blood and tissue basophils, inducing their degranulation - the release of histamine, serotonin and other vasoactive mediators (mediators of the inflammatory response). In addition, C5a is a chemoattractant for phagocytes; it attracts these cells to the site of inflammation;
C3b, C4b are opsonins, increase the adhesion of immune complexes to the membranes of macrophages, neutrophils, erythrocytes and thereby enhance phagocytosis.
Soluble receptors for pathogens. These are blood proteins that directly bind to various conservative, repeating carbohydrate or lipid structures of the microbial cell ( pattern-structures). These proteins have opsonic properties, some of them activate complement.
The main part of the soluble receptors are acute phase proteins. The concentration of these proteins in the blood rapidly increases in response to the development of inflammation due to infection or tissue damage. Acute phase proteins include:
C-reactive protein (it makes up the bulk of acute phase proteins), which received its name due to its ability
bind to phosphorylcholine (C-polysaccharide) of pneumococci. The formation of the CRP-phosphorylcholine complex promotes bacterial phagocytosis as the complex binds to Clg and activates the classical complement pathway. The protein is synthesized in the liver, and its concentration increases rapidly in response to interleukin-b;
Serum amyloid P is similar in structure and function to C-reactive protein;
Mannose-binding lectin activates complement via the lectin pathway and is one of the representatives of whey collectin proteins that recognize carbohydrate residues and act as opsonins. Synthesized in the liver;
Lung surfactant proteins also belong to the collectin family. They have opsonic properties, especially against unicellular fungi Pneumocystis carinii;
Another group of acute phase proteins consists of iron-binding proteins - transferrin, haptoglobin, hemopexin. Such proteins prevent the proliferation of bacteria that require this element.
Antimicrobial peptides. One such peptide is lysozyme. Lysozyme is a muromidase enzyme with a molecular weight of 14,000-16,000, which causes the hydrolysis of murein (peptidoglycan) of the bacterial cell wall and their lysis. Opened in 1909 by P.L. Lashchenkov, isolated in 1922 by A. Fleming.
Lysozyme is found in all biological fluids: blood serum, saliva, tears, milk. It is produced by neutrophils and macrophages (contained in their granules). Lysozyme has a greater effect on gram-positive bacteria, the basis of the cell wall of which is peptidoglycan. The cell walls of Gram-negative bacteria can also be damaged by lysozyme if they have previously been exposed to the membrane attack complex of the complement system.
Defensins and cathelicidins are peptides with antimicrobial activity. They are formed by the cells of many eukaryotes and contain 13-18 amino acid residues. To date, about 500 such peptides are known. In mammals, bactericidal peptides belong to the defensin and cathelicidin families. The granules of human macrophages and neutrophils contain α-defensins. They are also synthesized by epithelial cells of the intestines, lungs, and bladder.
Interferon family. Interferon (IFN) was discovered in 1957 by A. Isaacs and J. Lindeman while studying the interference of viruses (from lat. inter- between, ferens- carrier). Interference is a phenomenon where tissues infected with one virus become resistant to infection by another virus. It was found that such resistance is associated with the production of a special protein by infected cells, which was named interferon.
Currently, interferons are well studied. They are a family of glycoproteins with a molecular weight from 15,000 to 70,000. Depending on the source of production, these proteins are divided into type I and type II interferons.
Type I includes IFN α and β, which are produced by virus-infected cells: IFN-α by leukocytes, IFN-β by fibroblasts. In recent years, three new interferons have been described: IFN-τ/ε (trophoblast-derived IFN), IFN-λ and IFN-K. IFN-α and β are involved in antiviral defense.
The mechanism of action of IFN-α and β is not associated with a direct effect on viruses. It is caused by the activation in the cell of a number of genes that block the reproduction of the virus. The key link is the induction of the synthesis of protein kinase R, which disrupts the translation of viral mRNA and triggers apoptosis of infected cells through Bc1-2 and caspase-dependent reactions. Another mechanism is the activation of latent RNA endonuclease, which causes destruction of the viral nucleic acid.
Type II includes interferon γ. It is produced by T lymphocytes and natural killer cells after antigenic stimulation.
Interferon is constantly synthesized by cells; its concentration in the blood normally changes little. However, the production of IF increases when cells are infected with viruses or by the action of its inducers - interferonogens (viral RNA, DNA, complex polymers).
Currently, interferons (both leukocyte and recombinant) and interferonogens are widely used in clinical practice for the prevention and treatment of acute viral infections (influenza), as well as for therapeutic purposes in chronic viral infections(hepatitis B, C, herpes, multiple sclerosis, etc.). Since interferons have not only antiviral but also antitumor activity, they are also used to treat cancer.
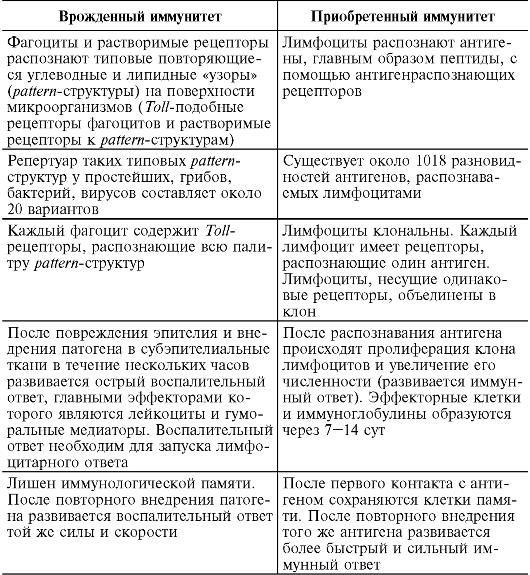
9.2.4. Features of innate and acquired immunity
Currently, factors of innate immunity are not usually called nonspecific. The barrier mechanisms of innate and acquired immunity differ only in the accuracy of tuning to “foreign”. Phagocytes and soluble innate immune receptors recognize “patterns,” and lymphocytes recognize the details of such a picture. Innate immunity is an evolutionarily more ancient method of defense, inherent in almost all living beings from multicellular organisms, plants to mammals due to the speed of reaction to the invasion of a foreign agent; it forms the basis of resistance to infection and protects the body from most pathogenic microbes. Only those pathogens that innate immunity factors cannot cope with include lymphocytic immunity.
The division of antimicrobial defense mechanisms into innate and acquired or pre-immune and immune (according to R.M. Khaitov, 200b) is conditional, since if we consider the immune process in time, then both are links in the same chain: first, phagocytes and soluble receptors for pattern- microbial structures, without such editing, the subsequent development of a lymphocytic response is impossible, after which lymphocytes again attract phagocytes as effector cells for the destruction of pathogens.
At the same time, dividing immunity into innate and acquired is advisable for a better understanding of this complex phenomenon (Table 9.2). The mechanisms of innate resistance provide quick protection, after which the body builds a stronger, layered defense.
Table 9.2. Features of innate and acquired immunity
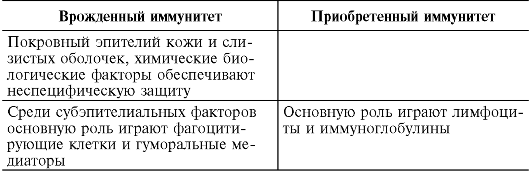 End of table. 9.2
End of table. 9.2
 Tasks for self-preparation (self-control)
Tasks for self-preparation (self-control)
Many substances and infections (microorganisms, viruses), penetrating the body, cause diseases. The internal enemies of the body are also dangerous - its dead cells, cancerous or infected with viruses. However, our body is able to protect itself from external and internal foreign agents. This ability is called immunity. Immunity is provided by the body's immune system. its components are leukocytes and organs in which they multiply and specialize (mature). This is red bone marrow thymus(thymus), spleen, The lymph nodes and lymphoid tissues located in the digestive, respiratory, and urinary systems. All leukocytes are formed in the bone marrow and mature in various organs. From them, leukocytes circulate together with blood and lymph and enter the tissues.
Leukocytes include several types of cells of various structures(lymphocytes, monocytes, eosinophils, etc.). Lymphocytes are divided into T lymphocytes (mature in the thymus) and B lymphocytes (mature in the bone marrow).
Significant number of leukocytes various types(53-81%) are capable of phagocytosis. More phagocytes are located in connective tissues kidneys, lungs, liver, skin.
Nonspecific innate immunity
The skin and mucous membranes prevent foreigners from entering the body. The peeling of dead skin epithelial cells and the movement of villi of the mucous membrane remove them, and destroy the bactericidal substances of the secretions of the sweat and sebaceous glands, epithelial mucus, etc.
If foreign agents have entered the body, phagocytes are sent to them and destroy the aggressors. In case of penetration large quantity foreigners or mass death of phagocytes in battle with them, the bone marrow accelerates the reproduction of such cells, and new forces become available to fight. This is how it works cellular immunity. It is closely related to humoral immunity factors - certain proteins that are constantly present in the blood. Some proteins attach to the membrane of the microorganism, indicating an alien - this facilitates phagocytosis. Others are involved in the destruction of the foreign cell membrane. Blood plasma proteins interferons help the body fight viruses.
Phagocytes And
Phagocytes And humoral immunity factors, which act in the first stage of the struggle, influence all aggressors in the same ways. These protection methods are inherited, i.e. congenital. Therefore, such immunity is called nonspecific innate.
Specific acquired immunity. The body cannot always cope with foreign agents without the help of specific immunity. Consequently, he uses other methods - those that act on aggressors in accordance with their characteristics. In these specific reactions of the body, two parts are also distinguished - humoral and cellular. Humoral immunity is carried out by B lymphocytes, and cellular immunity by T lymphocytes.
Humoral factors
Humoral factors specific immune reactions antibodies - immunoglobulin proteins. they are produced by B lymphocytes in response to antigens - substances that the body perceives as foreign. Usually these are certain proteins in the shells of the aggressors or toxins that they produce. B lymphocytes respond to each antigen by producing an antibody that corresponds specifically to that antigen. These proteins combine with the antigen and form antigen-antibody complexes—neutralization of both the antigen and the aggressor occurs.
Cellular link
Cellular link specific immunity destroys foreigners otherwise. Thus, T lymphocytes can attach to the membranes of cells affected by a virus or bacteria and destroy them.
Consequences of destructive activities T-lymphocytes are eliminated by phagocytes that devour neutralized aggressors and dead cells.
Lymphocytes circulating in the blood and lymph flow are activated only when they recognize an antigen. Their identification of the antigen that first entered the human body is a complex process that can last up to 14 days. Its consequence is an immune response, which usually involves T and B lymphocytes. B lymphocytes recognize the antigen and begin to synthesize antibodies to it. At the same time, B-lymphocytes, which are also sensitive to this antigen, multiply in the organs of the immune system. Some of them join the humoral attack, producing antibodies at enormous speed (up to 30,000 molecules per second). Other B lymphocytes become memory cells. T lymphocytes control the immune response by producing various interleukins. Interleukins can increase or decrease the activity of B lymphocytes, stimulate the proliferation of T lymphocytes and the formation of memory T cells, or suppress these processes.
Memory cells
Memory cells for months, and sometimes for years, they retain the ability to respond to the invasion of a “familiar” antigen. They do not waste time recognizing it, the immune response occurs immediately, and more antibodies are produced. This is how specific acquired immunity is formed.
Immune response
Immune response at the first meeting with infection is usually accompanied by feeling unwell person, fever, etc. If a person has acquired immunity to this infection, symptoms of the disease are not observed.
Artificial acquired immunity. There are infections, the first encounter with which can be fatal for a person. To create immunity against them, they are vaccinated - a vaccine is introduced into the human body. This is a small amount of killed or weakened pathogens or substances that are products of their vital activity. Such infection does not lead to illness. However, when a vaccine is administered, a full-fledged immune reaction develops: antibodies specific to this pathogen are produced and memory cells are formed. Therefore, after vaccination, the body encounters live pathogens, invading fully armed. Thus, through the introduction of vaccines, artificial acquired immunity is created.


