BIOCHEMISTRY OF LIPIDS
General characteristics of lipids
Lipids (from the Greek lipos - fat) are fats and fat-like substances. They are contained in all living cells and perform a number of vital important functions: structural, metabolic, energetic, protective, etc. Do not dissolve or slightly dissolve in water, dissolve well in organic solvents. Most of them are derivatives of alcohols, higher fatty acids or aldehydes.
Chemical properties and biological significance lipids are determined by the presence in their molecules of non-polar carbon chains and polar groups: -COOH, -OH, -NH 2, etc. This makes it possible for them to be surface-active, participate in the permeability of cell membranes, easily dissolve in organic solvents, be solvents for vitamins and other compounds.
There are two groups of lipids: simple and complex. Molecules simple lipid are formed from the remains of alcohols (glycerol, glycols, higher or cyclic) and higher fatty acids. it neutral fats, diol lipids, sterides and waxes. Molecules complex lipid consist of the remains of alcohols, higher fatty acids and other substances (nitrogenous bases, H 3 PO 4, H 2 SO 4, carbohydrates, etc.). Complex lipids include phosphatides, glycolipids, sulfatides. Often mono- and diglycerides, sterols, carotenes and other substances close to them are referred to lipids.
Neutral fats. They are a mixture of triglycerides - esters formed by trihydric alcohol glycerol and higher fatty acids.
Higher fatty acids are represented by saturated, unsaturated and cyclic carboxylic acids, and in some cases by hydroxy acids.
Saturated carboxylic acids usually have even number carbon atoms, for example:

Acids with an odd number of carbon atoms often have a branched carbon chain, such as isovaleric:
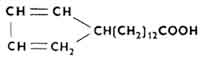
Unsaturated carboxylic acids can have one to four double bonds, for example:
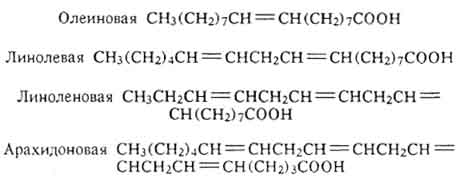
The composition of fats contains residues of cyclic acids, for example, haulmugric C 17 H 29 COOH, and hydroxy acids, for example:
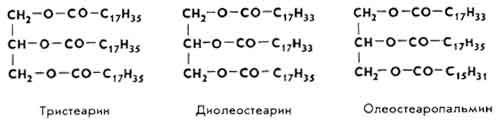
Triglycerides are simple and complex. A simple triglyceride molecule contains residues of one fatty acid, a complex triglyceride - two or three fatty acids:
Fats are widespread in nature. The composition of animal fats is dominated by residues
saturated fatty acids, which determines their solid consistency. Cow oil, lard, lamb and beef fats are of great importance. Fats vegetable origin they mainly contain residues of unsaturated fatty acids and are liquids (except for palmitic oil). The most commonly used oils are sunflower, olive, linseed, almond oil, etc.
Different foods and feeds contain different amounts of fat. In plants, they are usually concentrated in seeds, less in fruits. So, castor bean seeds contain 58-78% fat, rapeseed - 36-40, flax - 28.9-49, sunflower - 29-57, corn grains - 5, oats - 3, wheat 1-1.8%.
In the body of animals, fats are concentrated mainly in the subcutaneous tissue (up to 50%), the omentum, connective tissue capsules of the kidneys and genitals, in the liver and muscle tissue... Body fluids are poor in fats. Of these, milk has a relatively high percentage of fat (cow - 3.5%, reindeer - 17.1%). Fats are essential source chemical energy. So, with tissue oxidation of 1 g of fat, 9.3 kcal is formed (1 g of carbohydrates gives 4.3 kcal, proteins - 4.1 kcal). Fats are a source of endogenous water: when 100 g of fat is oxidized in tissues, 107.1 g of water are formed, which is very important for animals living in southern latitudes (for example, for camels) or for those that hibernate (for example, brown bears). Fats - solvents organic matter, especially fat-soluble vitamins. They take part in thermoregulation, as they have a low heat capacity, protect the body from mechanical damage(part of the capsules of the heart, kidneys, liver, eyes), determine the elasticity of the skin.
Distinguish between reserve (storage) and protoplasmic (structural) fats. The first of them are consumed by the body for various needs, which were mentioned above. The latter are constituent parts of cell membranes and are part of lipoprotein complexes.
Fats are food products for humans and animals. Vegetable oils can be used for
preparation of drying oil and varnishes. Many of them, in addition to food purposes and animal feeding (cakes), can be hydrogenated to produce various varieties of margarine. Fats from the liver of cod fish are used as a source of vitamins A and D. Technical fats are used in various areas of the national economy (in light, chemical and other industries).
The quality and purity of fats are characterized by physical and chemical constants (Table 3). Physical constants: density, melting and pour point, refractive index (for liquid fats); chemical constants: saponification number, Reichard - Meisl, iodine, acidic and some other indicators.
Saponification number is determined by the number of milligrams of KOH consumed to neutralize fatty acids that are formed during saponification of 1 g of fat.
Reichard - Meisl number characterized by the amount of 0.1 N. NaOH solution used to neutralize volatile fatty acids (butyric, nylon and caprylic) formed during hydrolysis of 5 g of fat and distilled off with steam.
Iodine number characterizes the presence of unsaturated fatty acids in the composition of fat and is determined by the number of grams of iodine capable of joining 100 g of fat.
Acid number indicates the presence of free fatty acids in the composition of fat, which
3. Physical and chemical constants of some fats
| Constants | Type of fat | ||
| beef | mutton | pork | |
| Density at 15 ° C, g / cm 3 | 0,923-0,933 | 0,932-0,961 | 0,931-0,938 |
| Melting point, ° C | 42-52 | 44-55 | 36-46 |
| Pour point, ° С | 27-38 | 32-45 | 26-32 |
| Refractive index (at 40 ° C) | 1,4510-1,4583 | 1,4566-1,4583 | 1,4536 |
| Saponification number | 190-200 | 192-198 | 193-200 |
| Reichard-Meisl number | I am | 0,3-0,9 | |
| Iodine number | 32-47 | 31-40 | 46-56 |
| Acid number | 0,1-0,6 | 0,1-0,2 | 0,3-0,9 |
are formed during the decomposition of its molecules. It is determined by the number of milligrams of KOH used to neutralize free fatty acids, which are contained in 1 g of fat.
The considered constants depend on the habitat, nutritional conditions, age, sex, breed of the animal, and on other factors. Thus, S. L. Ivanov found that animals living in northern latitudes have fats that are characterized by lower melting temperatures than animals of the same species kept in the south. In the composition of the fats of the former, residues of unsaturated fatty acids predominate, the latter - saturated.
Diol lipids. These lipids were discovered in the tissues of plants and animals by the Soviet scientist L. D. Bergelson in 1967-1973. They are a mixture of various esters formed from dihydric alcohols (ethanediol, propanediol, butanediol, etc.) and higher fatty acids. General formula
where n = 0, 1, 2, 3.
In the body, they perform the same functions as fats. Little has been studied.
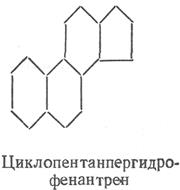
Sterides... Sterides are called esters of sterols and higher fatty acids (most often palmitic). Sterols, or sterols, are high molecular weight cyclic alcohols derived from cyclopentaneperhydrofenanthrene. The latter can be considered as a condensation product of hydrogenated phenanthrene and cyclopentane. Individual rings in are designated by letters (A, B, C, D), and the carbon atoms of the rings are designated by numbers.
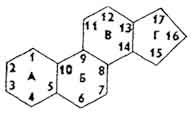
Sterols and sterols make up the unsaponifiable fraction of lipids and are part of cell membranes. V
in liver tissues, the steride content is about 50% of the total weight of all sterols. There are zoo-, phyto- and mycosterols. Derivatives of sterols are many steroid hormones (gonads and adrenal cortex), bile acids, D vitamins, steroid alkaloids, some triterpene antibiotics, toad skin gland poisons, and some carcinogenic substances. Sterols - crystalline substances, optically active, almost insoluble in water, soluble in organic solvents, colorless, able to sublimate, enter into chemical reactions typical for alcohols.
Of greatest interest are cholesterol and its derivatives - chrysterides, which are esters of cholesterol and higher fatty acids. Cholesterol was discovered in the 18th century. Konradi in the study of gallstones. There is a lot of it in the white matter of the brain. According to its chemical structure, cholesterol is a secondary cyclic alcohol.
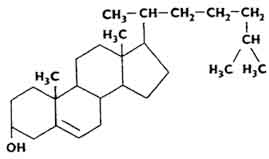
It is estimated that the human body weighing 70 kg contains about 140 g of cholesterol, of which 10% is concentrated in the adrenal glands, 2% in the nervous system, 0.25% in the bones. There is a lot of cholesterol in the liver (from 0.333 to 0.91% of the total mass). Cholesterol is able to retain a certain amount of water. Cholesterol forms complex compounds with proteins.
Sterols are excreted from the body mainly in the form of cholesterol (see above) and coprosterol.
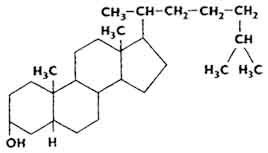
In the skin of animals and in the unsaponifiable fraction of lipids there is 7-dehydrocholesterol - a provitamin of vitamin D 3. Yeast contains ergosterol - a provitamin of vitamin D 2 (see chapter "Vitamins").
Waxes. Waxes - large group lipids, the molecules of which are formed from the residues of higher fatty acids and higher monohydric alcohols. The ratio of carbon in the acidic and alcoholic parts of the molecule is 1: 1 or 2: 1. Waxes contain impurities of free fatty acids and alcohols, hydrocarbons (C 27 - C 33) and fragrances. By origin, weights are distinguished by animals (bee, lanolin, spermaceti), vegetable (carnauba, candelilla), the excretion product of some insects (Chinese), fossils (ceresin and montan) and synthetic.
Beeswax . Produced by the wax glands of bees. Consists of a mixture of esters (up to 75%), free higher fatty acids and saturated hydrocarbons. Contains vitamin A and some other substances. The basis of the wax is an ester of myricyl alcohol and palmitic acid:
Beeswax does not dissolve in water, it will dissolve in chloroform and diethyl ether, gasoline and turpentine. It is the basis of the honeycomb. It is used for the preparation of ointments and plasters.
Lanolin. Received after washing sheep wool. It is a mixture of esters formed by higher alcohols (cetyl, carnauba, cholesterol, etc.) and higher fatty acids (lanopalmitic, myristic, etc.). By physical properties- it is a thick viscous mass of brown-yellow color with a weak odor, does not dissolve in water, dissolves in chloroform, ether, hygroscopic, does not saponify aqueous solutions alkalis, does not go rancid. They are used for the preparation of medicinal ointments and in cosmetics.
Spermaceti. This is a component of spermaceti oil, which is obtained from the brain of sperm whales. From one sperm whale, you can get 4-5 tons
spermacet. Its main component (up to 90%) is an ester of palmitic acid and cetyl alcohol:
Part of spermacet (10%) - esters of cetyl, stearic, oleic alcohols and lauric, myristic acids.
Spermaceti - white lamellar crystals, readily soluble in diethyl ether, acetone, hot ethanol, does not dissolve in water. It is used for the preparation of medicinal ointments and cosmetics. Used in the treatment of skin ulcers.
Vegetable waxes... Distributed in nature. Cover the leaves, stems, trunks and fruits of plants with a thin layer. Protect plant tissues from injury and microbes. Participate in the regulation of water exchange. They are a mixture of esters formed by higher alcohols (cetyl, myricyl) and fatty acids (cerotinic, carnauba, montanic, stearic, palmitic, oleic). Carnauba wax is widely used for making candles, etc. It is obtained from the leaves of some palm trees. The basis of the wax is an ester of myricyl alcohol and cerotinic acid:
Phosphatides... The phosphatide molecule is formed by residues of higher alcohols and higher fatty acids, phosphoric acid and a nitrogenous base. With other lipids and proteins, they form the chemical basis of cell membranes, determine their selective permeability for various substances, participate in the processes of cellular respiration and electron transfer.
The phosphatide molecule usually consists of two parts: polar (hydrophilic) and apolar
(hydrophobic). The hydrophilic "head" has negative charge phosphate and positive nitrogen, being a permanent dipole (zwitterion). The hydrophobic tail consists of long chains of higher fatty acid residues. It is this molecular structure that determines the surface-active properties of the lipid, makes it possible to form film structures in a monolayer at the interface, interact with various (polar and apolar) compounds, and actively participate in assimilation and dissimilation reactions.
Most phosphatides are found in the nervous tissue (up to 26-30% of dry weight), liver (16%), kidneys (11%) and heart (10%). They are synthesized in the Golgi complex.
Distinguish between glycero-, inositol- and sphingosine phosphatides.
Glycerophosphatides
Lecithins, or folin phosphatides... In the formation of molecules α - and β -lecithins involve glycerol, saturated and unsaturated higher fatty acids, H 3 PO 4 and choline. V α -lecithin, the choline residue and H 3 PO 4 are located near the C 1 atom of the alcohol molecule.

A lot of lecithin is found in the tissues of the spinal cord and brain (35.2-12.4%), yolk chicken eggs(6.5-12%), lungs, myocardium, kidneys (5.9-5.2%), etc. It is used by the body for the biosynthesis of acetidcholine. It is used internally (in the form of pills) in the treatment of diseases nervous system, anemias, general loss of strength.
Many vegetable feeds are also rich in lecithin: sunflower seeds (38.5%), flax (36.2%), soybeans (35%), etc.
Mullets, or colamine phosphatides... The cephalin molecules contain ethanolamine (colamine).

The cephalin fraction is the lipid base of human brain tissue (66%), cattle liver (51%), myocardium (30%), and chicken egg yolk (28.7%). Rich in mullet soybeans (65%), cotton seeds (71.2%), flax and sunflower (61.5%). Cephalins form lipoprotein complexes with proteins. Many of them are found in mitochondria.
Serine phosphatides. In the serine phosphatide molecule, the nitrogenous base is the amino acid of the series.
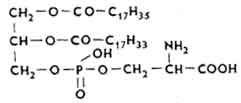
Serine phosphatides are abundant in the nervous tissue, liver, kidneys and other organs. These are proto-gasmatic lipids. There are many of them in mitochondria.
There is a genetic relationship between lecithins, cephalins and serine phosphatides, since nitrogenous bases can pass into each other:
Acetal phosphatides(plasmalogens). Aldehydes of higher fatty acids are involved in the structure of acetal phosphatides. Most often, acetal phosphatides have the following structure:
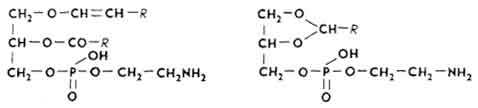
They differ from each other in nitrogenous bases, higher fatty acids and their aldehydes, as well as in the methods of formation of acetals. Make up about 12% of all tissue phosphatides. The ethanolamine-cephalin fraction of the brain is 2/3 composed of acetal phosphatides; sperm by 55-60%. In some organs (liver, myocardium, kidneys, muscles), the content of acetal phosphatides increases with age.
Cardiolipins. First isolated from myocardial extract. The basis of their molecule is made up of three glycerol residues, interconnected by phosphodiester bonds of type 1,3 (R - residues of higher fatty acids).
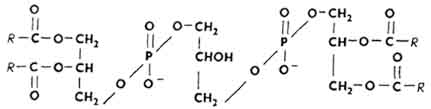
Cardiolipins occupy almost 10% of all mitochondrial lipids. These lipids are involved in oxidative phosphorylation and electron transfer, in complement binding during blood clotting.
Inositrophosphatides
Their molecule is an ester formed by glycerol, higher fatty acids, H 3 PO 4 and inositol hexahedral alcohol. Distinguish between monophosphoinositides and diphosphoinositides.
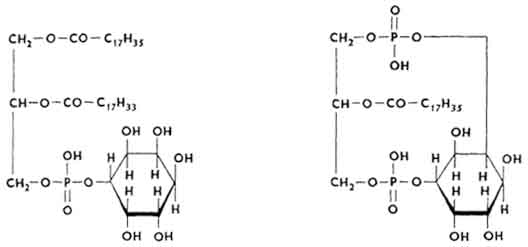
Many inositol phosphatides are found in the nervous tissue (brain), especially in the myelin sheaths of nerve fibers. Inositol phosphatides are able to form
complex compounds with proteins. The remainder of inositol can react with galactose, tatronic acid and higher fatty acids, colamine, combining metabolic products of proteins, carbohydrates and lipids characteristic of nervous tissue into a single whole.
Sphingosine Phosphatides
Sphingosine phosphatide molecules are formed from sphingosine residues, higher fatty acids, phosphoric acid and choline.

They are often called sphingomyelins. They are rich in nerve tissue (they form the basis of the myelin sheaths of nerve fibers), spleen, lungs, kidneys, pancreas. Sometimes the lipid molecule contains a dihydrosphingosine residue. Sphingosine phosphatides are white crystalline substances that form an aqueous colloidal solution. Higher fatty acids are represented by stearic acid (50%), less - lignoceric acid and nerve acid. Make up 20% of all brain lipids.
Glycolipids... These are fat-like substances, the molecules of which also contain a carbohydrate component.
Cerebrosides. They are a mixture of esters built from sphingosine residues, higher fatty acids and galactose. In cerebrosides, sphingosine is contained in the form of cerebron, a compound with cerebronic acid and galactose, kerazine, a compound with lignoceric acid and galactose, and nervone, a compound with neronic acid and galactose (see below).
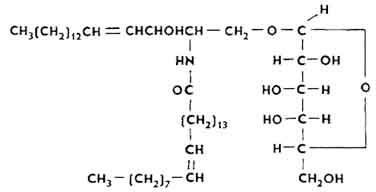
There are many cerebrosides in brain tissues. As part of the spleen molecule, they contain glucose residues (glucocerebrosides).
Cerebrosides are solids that do not dissolve in water, dissolve in diethyl and petroleum ethers, swell when boiled, and decompose when heated to 200 ° C. They perform structural and metabolic functions in the body.
Gangliosides... The ganglioside molecule contains on average 40-43% galactose, 21% neuraminic acid, 13% sphingosine, 23-26% hexosamines, glucose and stearic acid. There are many lipids in the nervous tissue, parenchymal organs, and blood cells. Gangliosides - structural components neurons, neutralize poisons, participate in the conduction of nerve impulses, etc.
Sulfatides... These are esters formed by sphingosine, cerebronic or lignoceric acid, galactose and sulfuric acid.
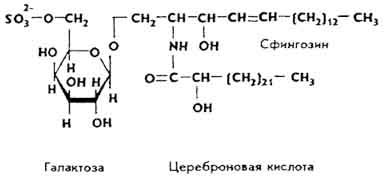
Sulfatides are found in the tissues of the brain, liver, kidneys, muscles, etc. They appear in the urine with cerebral sclerosis.
Acylglycerols, or neutral lipids - the most common group of lipids in nature. These compounds are esters of fatty acids and a trihydric alcohol of glycerol (glycerides), in which one, two or three hydroxyl groups of glycerol can be esterified to form, respectively mono-, di- and triacylglycerols:
Triacylglycerols are most commonly found in nature. Since all of the above acylglycerols do not contain ionic groups, they belong to neutral lipids. If all three acid radicals belong to the same fatty acid, then such triacylglycerols are called simple, if they are different fatty acids, then they are called mixed.
The fatty acids that make up the triacylglycerols determine their physicochemical properties. The more residues of short-chain and unsaturated acids in lipids, the lower the melting point and the higher the solubility. For example, animal fats usually contain a significant amount of saturated fatty acids, due to which they remain solid at room temperature. Fats, which contain many unsaturated acids, will be liquid under these conditions; they are called oils.
Most animal fats contain in various proportions esters of palmitic, stearic, palmitooleic, oleic and linoleic acids. Human fat melting at 15 ° C contains about 70% unsaturated fatty acids, and at body temperature it is in a liquid state. Fats from different tissues of one organism, like vegetable oils, can differ from each other both in the length of hydrocarbon chains and in the degree of their unsaturation.
Constants are used to characterize the properties of fat, or fat numbers- acid number, saponification number, iodine number.
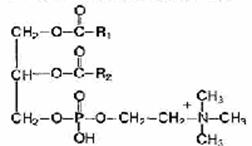
The common structural fragment of all phosphoglycerides is phosphatidic acid (1,2-diacyl, 3-phosphoglycerol).
Phosphatidic acid is formed in the body during the biosynthesis of triacylgl and nerols and phosphoglycerides as a common intermediate metabolite; in tissues, it is present in small quantities. It should be noted that all natural phosphoglycerides belong to the L-series. Various phosphoglycerides differ from each other by additional groups attached by a phosphoester bond to phosphatidic acid, i.e. R3. The fatty acid composition of various phosphoglycerides differs even within the same organism and, along with substituent groups, determines the specificity of phospholipids:
Phosphatidylcholine (lecithin). It contains the amino alcohol choline (3-hydroxyethyltrimethylammonium hydroxide):
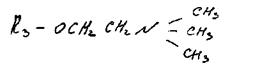 |
Phosphatidylethanolamine (cephalin). Instead of choline, the composition of phosphatidylethanolamines contains the nitrogenous base ethanolamine HO-CH 2 -CH 2 -NH 3.
In the body of animals and in higher plants, phosphatidylcholines and phosphatidylethanolamines are found in greatest quantities. These two groups of glycerophospholipids are the main lipid components of cell membranes.
Phosphatidylinositols Unlike other groups of phosphoglycerides, the composition of phosphatidylinositols instead of nitrogen-containing compounds includes the 6-carbon cyclic alcohol inositol, represented by one of its stereoisomers, monositol.
Phosphatidylglycerols. Like phosphatidylinositols, phosphatidylglycerols do not contain nitrogen-containing compounds. In these compounds, another glycerol molecule serves as a polar group.
Neutral fats include a group of lipids consisting of a triatomic alcohol - glycerol and three residues of fatty acids, therefore they are called triglycerides.
The composition of neutral fats can contain the same fatty acids, such as palmitic. In this case, an ester is formed - triglyceride, tripalmitin. These are simple fats. If the fats contain residues of different fatty acids, then mixed fats are formed.
This reaction equation shows the reversible processes of synthesis (upper arrow) and hydrolysis (lower) of fat.
Natural fats are distinguished by a wide variety of fatty acids included in their composition, their different arrangement in the molecule and the degree of unsaturation. There are potentially millions of triglyceride isomers.
Fatty acids are organic acids with a long hydrocarbon chain (radical R) containing from 4 to 24 or more carbon atoms and one carboxyl group. The general formula of fatty acids is
CnH2n + 1COOH, or R-COOH.
Many fatty acids are characterized by the presence of an even number of carbon atoms, which is apparently due to their synthesis by adding two-carbon units to the growing hydrocarbon chain.
Fatty acids with 16 or 18 carbon atoms, which are called higher fatty acids, are most often part of the fats of the human body. Higher fatty acids are divided into saturated saturated) and unsaturated (unsaturated)
In saturated fatty acids, all free bonds of carbon atoms are filled with hydrogen. Such fatty acids do not have double or triple bonds in the carbon chain. Unsaturated fatty acids have double bonds in the carbon chain (-C = C-), the first of which occurs between the ninth and tenth carbon atoms from the carboxyl group. Fatty acids with triple bonds are rare. Fatty acids containing two or more double bonds are called polyunsaturated.
With an increase in the number of carbon atoms in fatty acid molecules, their melting point increases. Fatty acids can be solids(for example, stearic) or liquid (for example, linoleic, arachidonic); they are insoluble in water and very slightly soluble in alcohol.
Solid fats are fats of animal origin, with the exception of fish oil... Liquid fats are vegetable oils, with the exception of coconut and palm oils, which solidify when cooled. In the body of animals and plants, unsaturated fatty acids are twice as large as saturated ones.
Unsaturated fatty acids are more reactive than saturated ones. They easily attach two hydrogen atoms at the site of double bonds, turning into saturated ones:
This process is called hydrogenation. Substances subjected to hydrogenation change their properties. For example, vegetable oils are converted to solid fat. The hydrogenation reaction is widely used to obtain solid edible fat - margarine from liquid vegetable oils.
Polyunsaturated fatty acids are of particular importance to humans. They are not synthesized in the body. With their lack or absence in food, the metabolism of fats, in particular cholesterol, is disturbed, pathological changes are observed in the liver, skin, and platelet function. Therefore, unsaturated fatty acids such as linolenic and linoleic are essential nutritional factors.
In addition, they promote the release of fats from the liver, which are synthesized in it, and prevent obesity. This action of unsaturated fatty acids is called the lipotropic effect. Unsaturated fatty acids serve as precursors for the synthesis of biologically active substances- prostaglandins. The daily requirement of a person for polyunsaturated acids is normally about 15 g.
Neutral fats accumulate in fat cells (adipocytes), under the skin, in the mammary glands, fat capsules around internal organs abdominal cavity; a small amount is found in skeletal muscles. The formation and accumulation of neutral fats in adipose tissues is called deposition. Triglycerides form the basis of reserve fats, which are the body's energy reserve and are used during fasting, insufficient fat intake, and prolonged physical exertion.
Neutral fats are also found in cell membranes, complex proteins protoplasm and are called protoplasmic. Protoplasmic fats are not used as an energy source even when the body is depleted, since they perform a structural function. Their number and chemical composition are constant and do not depend on the composition of food, while the composition of reserve fats is constantly changing. In humans, protoplasmic fats account for about 25% of the total body fat mass (2-3 kg).
In various cells of the body, especially in adipose tissue, enzymatic reactions of biosynthesis and disintegration of neutral fats constantly occur:
During the hydrolysis of fats in the body, glycerol and free fatty acids are formed. This process is catalyzed by lipase enzymes. The process of hydrolysis of fats in the tissues of the body is called lipolysis. The rate of lipolysis increases significantly with physical endurance exercise, and the activity of lipases increases during training.
If the fat decomposition reaction is carried out in the presence of alkalis (NaOH, KOH), then sodium or potassium salts of fatty acids are formed, which are called soaps, and the reaction itself is saponification. This chemical reaction underlies the production of soap from various fats and their mixtures.
Phospholipids
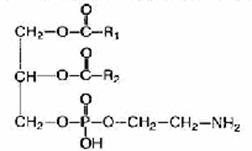
Phospholipids are fat-like substances consisting of alcohol (usually glycerol), two residues of fatty acids, a phosphoric acid residue and a nitrogen-containing substance (amino alcohol - choline or colamine).
If choline is included in the phospholipid molecules, they are called lecithins, and if colamine - cephalins.
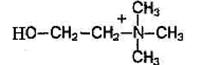
 Choline Colamine
Choline Colamine
Alpha Lecithin Alpha Cephalin
The structure of beta isomers differs in that the residues of phosphoric acid and amino alcohol are located at the second (middle) carbon atom of glycerol.
Phosphatides, especially lecithin a large number contained in the yolk of eggs. In the human body, they are widely distributed in the nervous tissue. Phospholipids play an important biological role, being a structural component of all cell membranes, suppliers of choline, which is necessary for the formation of a neurotransmitter, acetylcholine. Phospholipids depend on such membrane properties as permeability, receptor function, catalytic activity of membrane-bound enzymes.
Phospholipids dominate membranes animal cell, they are also contained in many of its subcellular particles.
The biological role of phospholipids in the body is significant and varied. As an indispensable component biological membranes fofolipids take part in their barrier, transport, receptor functions, in the division of the inner space of the cell into cellular organelles - "cisterns", compartments. These functions of membranes are currently considered to be the most important regulatory mechanisms of cell activity. The presence of phospholipids in membranes is also necessary for the functioning of membrane-bound enzyme systems.
STEROIDS
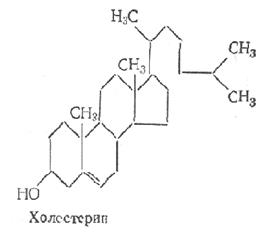
Steroids are classified as unsaponifiable lipids. By their chemical nature, steroids are derivatives of cyclopentaneperhydrofenanthrene. They are divided into sterols and sterols. Sterols are high molecular weight cyclic alcohols with a nucleus in the molecule.
 |
Various tissues also contain sterides - esters formed by sterols and fatty acids. Sterols and their derivatives perform various functions in the body. Cholesterol is of great biological importance in the animal organism. Violation of his exchange may entail pathological changes vessels - atherosclerosis. Cholesterol serves as a biological precursor of bile acids, steroid hormones. Bile acids have great importance in the process of lipid breakdown in the intestine. Steroid hormones regulate numerous metabolic processes.
PROTEINS
The most important compounds in every organism are proteins. They are necessarily found in all cells of the body; in most of them, protein accounts for more than half of the dry residue. All major manifestations of life are associated with proteins. “Life,” wrote F. Engels, “is a way of existence of protein bodies ... Wherever we meet life, we find that it is connected with some protein body, and everywhere we meet some protein body, that is not in the process of decay, we, without exception, meet the manifestations of life. "
Proteins - high-molecular nitrogen-containing organic compounds consisting of amino acid residues. In the composition of some proteins, along with amino acids, other compounds are also found.
Living organisms are characterized by a wide variety of proteins that form the basis of the structure of the body and provide many of its functions. It is believed that there are approximately 1010-1012 different proteins in nature, which explains the large variety of living organisms. In unicellular organisms, there are about 3,000 different proteins, and in the human body - about 5,000,000.
Despite the complexity of their structure and diversity, all proteins are built from relatively simple structural elements - amino acids. Proteins are polymeric molecules containing 20 different amino acids. A change in the number of amino acid residues and the sequence of their arrangement in a protein molecule provides the possibility of the formation of a huge amount of proteins that differ in their physicochemical properties, structural or functional role in the body.
For any organism, proteins play a decisive role in all life processes. Associated with them are such properties of a living organism as irritability, contractility, digestion, the ability to grow, reproduce, and move. Consequently, squirrels are the main carriers of life. In inanimate nature, compounds like proteins are not found.
Chemical composition and biological role of proteins
Proteins are high-molecular nitrogen-containing substances, the hydrolysis of which produces amino acids. Sometimes proteins are called proteins (from the Greek proteus - the first, main), thus determining their most important role in the life of all organisms. Protein in the human body accounts for an average of 45% of dry body weight (12-14 kg). Its content in individual tissues is different. The largest amount of protein is found in muscles, bones, skin, digestive tract and other dense tissues.
The daily protein requirement of an adult who is not involved in sports is on average 1.3 g per 1 kg of body weight, or about 80 g. With high energy consumption, the need for them increases by about 10 g for every 2100 kJ of increasing energy consumption ...
Proteins enter the body mainly with food of animal origin. Plants contain significantly less proteins: in vegetables and fruits - only 0.3-2.0% of the mass of fresh tissue; the largest amount of proteins - in legumes - 20-30%, cereals - 10-13 and mushrooms - 3-6%.
Elemental composition of proteins. The most important chemical elements of all proteins are carbon (50-55%), oxygen (21-23%), hydrogen (6.5-7.3%), nitrogen (15-18%), sulfur (0.3- 2.5%). The proteins also contain phosphorus, iron, iodine, copper, manganese and other chemical elements.
Fats (synonym: neutral fats, triglycerides) are esters of the trihydric alcohol of glycerol and higher or medium fatty acids, the main constituent of animal fats and vegetable oils, are present in all animal and plant tissues, are one of the main nutrients... Zh., Used in human nutrition, it is more correct to call fatty foods, because in addition to fats proper, they also include fat-like substances - lipids (sterols, phospholipids, etc.). Physicochemical properties Zh. Are determined by the nature of the residues of fatty acids in their molecule. Iron containing significant amounts of saturated fatty acids (palmitic, stearic, etc.), have more high temperature melting; Zh., Which include many mono- and polyunsaturated fatty acids, are in a liquid state at ordinary temperatures and are called oils. Vegetable oils with a high content of polyunsaturated fatty acids (linseed, hemp, poppy, tung oil) are known as drying oils. under the influence of atmospheric oxygen, they polymerize and harden.
The biological usefulness of iron is determined by the presence in their composition of fat-soluble vitamins A, D, and E (tocopherols), polyunsaturated fatty acids (linoleic, linolenic, arachidonic), phospholipids (lecithin, sphingomyelin), sterols (b-sitosterol), etc., as well as ease of absorption in the gastrointestinal tract. Zh. Dissolve well in organic solvents - benzene, chloroform, ether, carbon disulfide, petroleum ether, hot alcohol (cold alcohol is more difficult), acetone and do not dissolve in water. When added to the liquid in water, surfactants - detergents, they are able to form fat emulsions. Neutral fats enter into all chemical reactions characteristic of esters (products of substitution of hydrogen atoms in OH groups of mineral or carboxylic acids) and, above all, into the saponification reaction, as a result of which glycerol and fatty acids are formed from triglycerides. Saponification of iron can occur both during catalytic hydrolysis and by the action of acids or alkalis on the liquid.
To obtain fats of a more solid consistency from vegetable oils, which are used as a fat base in the production of margarines, hydrogenation (hydrogenation) is used, i.e. saturation of the molecules of these oils with hydrogen. During storage, especially in the light and with free access of air, fats acquire bad taste- go rancid. It has been established that oxidation of unsaturated fatty acids with atmospheric oxygen plays the main role in the rancidity of iron. The resulting peroxides decompose to form aldehydes. Oxidation of unsaturated fatty acids to b-keto acids can also occur (the so-called ketone rancidity of fats). The criteria for the properties of fats are their acid number, saponification number, iodine number and peroxide number. Acid number (CN) is used to estimate the amount of fatty acids contained in fat as impurities in a free state; numerically, it is equal to the number of milligrams of caustic potassium KOH spent to neutralize one gram of Zh.
The saponification number (CHR) is the number of milligrams of potassium hydroxide consumed to neutralize all fatty acids (both free and triglycerides) contained in 1 g of fat; OR serves to estimate the total amount of fatty acids in the fat under study. The value of CHO of the main animal fats (beef, lamb, pork) is practically the same - 191-206. The iodine number (ID) is used to determine the total amount of unsaturated compounds present in the fat, and is numerically equal to the amount of iodine added under standard conditions to 100 parts by weight of fat. The YCh of beef fat is 32-47, lamb fat 35-46, pork fat 46-66. The relative content of peroxides of fatty acids in the fat under study is evidenced by the peroxide number (PN), which is determined by titration of free iodine released when potassium iodide is added to the fat; IF is expressed as a percentage of iodine by weight. Preparations of fats labeled with radionuclides (most often radioactive iodine) are used for radioisotope diagnostics, for example, for diseases gastrointestinal tract... Such diagnostic drugs are 131I-glycerol trioleate (131I-triolein) and 131I-vegetable oils (sunflower, corn and olive).
Diagnosis of disturbances in the processes of digestion and absorption of iron with the help of fats containing a radioactive label is based on the fact that neutral fat, before absorption in the intestine, undergoes splitting under the action of pancreatic lipase, while fatty acids are absorbed directly. Therefore, in diseases of the pancreas, the absorption of neutral fat, for example, 131I-triolein, is impaired during the normal absorption of 131I-oleic acid, and in diseases of the intestine, the absorption of both triolein and free oleic acid decreases. The study of the absorption of labeled Zh. Serves as a simple and reliable method for detecting steatorrhea; it makes it possible to distinguish steatorrhea of pancreatogenic origin from steatorrhea, the cause of which is impaired absorption of triglycerides and fatty acids in diseases of the small intestine.
The pathology of fat metabolism in a number of diseases is detected by determining the quantitative and qualitative composition of lipids in the blood, incl. neutral F. Normally, blood serum contains up to 2.3 mmol / l (200 mg / 100 ml) of neutral fats, or triglycerides. The content of neutral iron in the blood (lipemia) varies significantly depending on the timing of food intake, especially fatty food. In blood healthy person hyperlipemia is noted 2-3 hours after the fat load, reaches a maximum after 4-6 hours, and after 8-9 hours the content of iron returns to its original value. Therefore, the diagnostic determination of the total content of triglycerides and other lipids in the blood should be carried out on an empty stomach. An increased concentration of neutral iron in the blood serum testifies to the suppression of the mechanisms of utilization of iron.
Hyperlipemia is observed in obesity, hepatitis, atherosclerosis, nephrosis, diabetes mellitus and blockade of the mononuclear phagocyte system. It is an unfavorable biochemical symptom, because the increased content of iron in the blood serum contributes to the suppression of the synthesis of fatty acids and the partial transfer of the pathways for the utilization of cellular funds of acetyl-CoA in the direction of the biosynthesis of cholesterol. An increase in the concentration of triglycerides containing saturated fatty acid residues in their molecule is especially unfavorable in this respect. Neutral iron (triglycerides) in the clinic are determined by the Carlson-Ignatovskaya method, based on measuring the amount of glycerol released as a result of triglyceride hydrolysis. A person's need for living depends on age, the nature of work, and climatic conditions... On average, the need for iron is 80-100 g per day. In old age, as well as at low physical activity and in mental labor, the demand for iron decreases, and in a cold climate, the demand for iron increases. Excessive consumption of animal fat is a risk factor for the development of atherosclerosis. Lack of fat in food or a constant violation of their optimal ratio leads to various metabolic and energy disorders and is the cause of a number of diseases.
Molecules of phospholipids and glycolipids are amphiphilic, that is, the hydrocarbon radicals of fatty acids and sphingosine are hydrophobic, and the other part of the molecule formed from carbohydrates, the phosphoric acid residue with choline, serine, ethanolamine attached to it, is hydrophilic. As a result, in the aqueous medium, the hydrophobic regions of the phospholipid molecule are displaced from the aqueous medium and interact with each other, and the hydrophilic regions are in contact with water, resulting in the formation of a double lipid layer of cell membranes (Figure 9.1.). This double membrane layer is permeated with protein molecules - microtubules. Oligosaccharides are attached to the outside of the membrane. The amount of protein and carbohydrates in different membranes is not the same. Membrane proteins can perform structural functions, can be enzymes, carry out transmembrane transport of nutrients, and can perform various regulatory functions. Membranes always exist as closed structures (see Figure 9.1). The lipid bilayer is self-assembling. This ability of membranes is used to create artificial lipid vesicles - liposomes.
Liposomes are widely used as capsules for the delivery of various drugs, antigens, enzymes to various organs and tissues, since lipid capsules are able to penetrate cell membranes. This allows drugs to be directed exactly to the target in the affected organ.
Figure 9.1. Scheme cell membrane from a double lipid layer. The hydrophobic regions of the lipid molecule are attracted to each other; hydrophilic regions of the molecule are on the outside. Protein molecules permeate the lipid bilayer.
Lipid metabolism
In the body, neutral fats are found in 2 forms: storage fat and protoplasmic fat.
The protoplasmic fat contains phospholipids and lipoproteins. They are involved in the formation of the structural components of cells. The membranes of cells, mitochondria and microsomes are composed of lipoproteins and regulate the permeability of individual substances. The amount of protoplasmic fat is stable and does not change with fasting or obesity.
Reserve (reserve) fat - it contains triacylglycerols of fatty acids - is found in the subcutaneous fatty tissue and in the fat depots of internal organs.
The function of reserve fat is that it is a reserve source of energy available for use during fasting; it is an insulating material against cold and mechanical injuries.
It is also important that lipids, when decaying, release not only energy, but also a significant amount of water:
When 1 gram of protein is oxidized, 0.4 g is released; carbohydrates - 0.5 g; lipids - 1 g of water. This property of lipids is of great importance for animals living in the desert (camels).
Digestion of lipids in the gastrointestinal tract
In the oral cavity, lipids are only mechanically processed. There is a small amount of lipase in the stomach, which hydrolyzes fats. Low activity of lipase of gastric juice is associated with the acidic reaction of the contents of the stomach. In addition, lipase can only affect emulsified fats; there are no conditions in the stomach for the formation of fat emulsions. Only in children and monogastric animals does gastric acid lipase play an important role in lipid digestion.
The intestine is the main site for the digestion of lipids. V duodenum lipids are affected by liver bile and pancreatic juice, while the intestinal contents (chyme) are neutralized. Fats are emulsified by bile acids. Bile contains: cholic acid, deoxycholic (3.12 dihydroxycholanic), chenodeoxycholic (3.7 dihydroxycholanic) acids, sodium salts of paired bile acids: glycocholic, glycodeoxycholic, taurocholic, taurodeoxycholic. They are composed of two components: cholic and deoxycholic acids, as well as glycine and taurine.
deoxycholic acid chenodeoxycholic acid
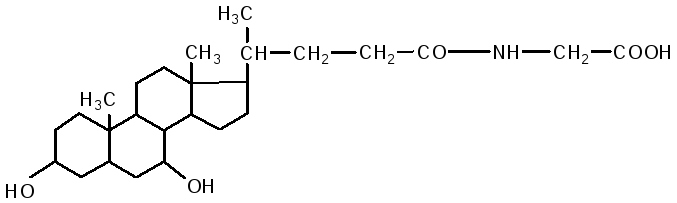
glycocholic acid
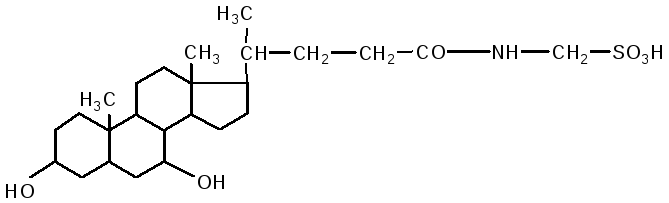
taurocholic acid
Bile salts emulsify fats well. This increases the area of contact of enzymes with fats and increases the effect of the enzyme. Insufficient synthesis of bile acids or delayed intake disrupts the effectiveness of enzymes. Fats are usually absorbed after hydrolysis, but some of the finely emulsified fats are absorbed through the intestinal wall and pass into the lymph without hydrolysis.
Esterases break the ester bond in fats between the alcohol group and the carboxyl group of carboxylic acids and inorganic acids (lipase, phosphatase).
Lipase hydrolyzes fats into glycerol and higher fatty acids. Lipase activity is increased by the action of bile, i.e. bile directly activates lipase. In addition, the activity of lipase is increased by Ca ++ ions due to the fact that Ca ++ ions form insoluble salts (soaps) with liberated fatty acids and prevent their suppressive effect on lipase activity.
Under the action of lipase, at the beginning, ether bonds are hydrolyzed at the α and α 1 (side) carbon atoms of glycerol, then at the β-carbon atom:
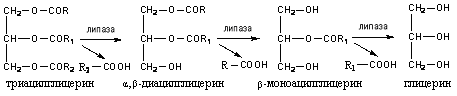
Under the action of lipase, up to 40% of triacylglycerides are broken down to glycerol and fatty acids, 50-55% is hydrolyzed to 2-monoacylglycerols and 3-10% is not hydrolyzed and absorbed as triacylglycerols.
The sterides of the feed are broken down by the enzyme cholesterol esterase to cholesterol and higher fatty acids. Phosphatides are hydrolyzed under the influence of phospholipases A, A 2, C and D. Each enzyme acts on a specific ester bond of the lipid. The points of application of phospholipases are shown in the diagram:
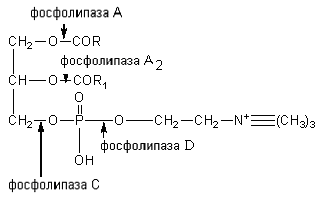
Phospholipases of the pancreas, tissue phospholipases are produced in the form of enzymes and are activated by trypsin. Phospholipase A 2 of snake venom catalyzes the elimination of unsaturated fatty acid at position 2 of phosphoglycerides. In this case, lysolecithins with hemolytic action are formed.
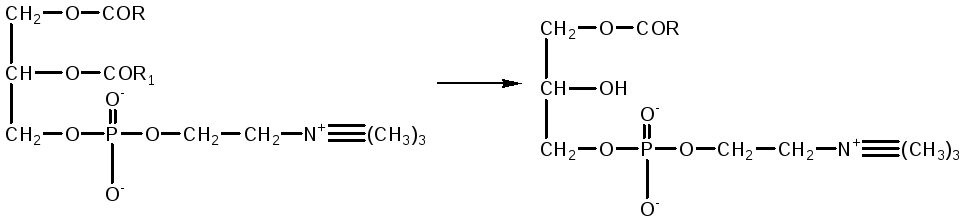
phosphotidylcholine lysolecithin
Therefore, when this poison enters the bloodstream, severe hemolysis occurs. In the intestine, this danger is eliminated by the action of phospholipase A1, which rapidly inactivates lysophosphatide as a result of the cleavage of a saturated fatty acid residue from it, converting it into inactive glycerophosphocholine.
Lysolecithins in low concentrations stimulate the differentiation of lymphoid cells, the activity of protein kinase C, and enhance cell proliferation.
Colamine phosphatides and serine phosphatides are cleaved by phospholipase A to lysocolamine phosphatides, lysoserine phosphatides, which are further cleaved by phospholipase A 2 . Phospholipases C and D hydrolyze choline bonds; colamine and serine with phosphoric acid and the remainder of phosphoric acid with glycerol.
Lipid absorption occurs in the small intestine. Fatty acids with a chain length of less than 10 carbon atoms are absorbed in unesterified form. For absorption, the presence of emulsifying substances is necessary - bile acids and bile.
Resynthesis of fat characteristic of a given organism occurs in the intestinal wall. The concentration of lipids in the blood is high within 3-5 hours after ingestion. Chylomicrons- small particles of fat, formed after absorption in the intestinal wall, are lipoproteins surrounded by phospholipids and a protein membrane, inside they contain molecules of fat and bile acids. They enter the liver, where lipids undergo intermediate metabolism, and bile acids pass to gall bladder and back into the intestines (see Figure 9.3 on page 192). As a result of this circulation, a small amount of bile acids is lost. It is believed that a molecule of bile acid makes 4 circuits per day.


