Molecules of phospholipids and glycolipids are amphiphilic, that is, the hydrocarbon radicals of fatty acids and sphingosine are hydrophobic, and the other part of the molecule formed from carbohydrates, the phosphoric acid residue with choline, serine, ethanolamine attached to it, is hydrophilic. As a result, in the aqueous medium, the hydrophobic regions of the phospholipid molecule are displaced from the aqueous medium and interact with each other, and the hydrophilic regions are in contact with water, resulting in a double lipid layer cell membranes(Figure 9.1.). This double membrane layer is permeated with protein molecules - microtubules. Oligosaccharides are attached to the outside of the membrane. The amount of protein and carbohydrates in different membranes is not the same. Membrane proteins can perform structural functions, can be enzymes, carry out transmembrane transport of nutrients, and can perform various regulatory functions. Membranes always exist as closed structures (see Figure 9.1). The lipid bilayer is self-assembling. This ability of membranes is used to create artificial lipid vesicles - liposomes.
Liposomes are widely used as capsules for the delivery of various drugs, antigens, enzymes to various organs and tissues, since lipid capsules are able to penetrate cell membranes. This allows drugs to be directed exactly to the target in the affected organ.
Figure 9.1. Diagram of a cell membrane from a lipid bilayer. The hydrophobic regions of the lipid molecule are attracted to each other; hydrophilic regions of the molecule are on the outside. Protein molecules permeate the lipid bilayer.
Lipid metabolism
In organism neutral fats are in 2 forms: storage fat and protoplasmic fat.
The protoplasmic fat contains phospholipids and lipoproteins. They are involved in the formation of the structural components of cells. The membranes of cells, mitochondria and microsomes are composed of lipoproteins and regulate the permeability of individual substances. The amount of protoplasmic fat is stable and does not change with fasting or obesity.
Reserve (reserve) fat - it contains triacylglycerols of fatty acids - is found in the subcutaneous fatty tissue and in fat depots internal organs.
The function of reserve fat is that it is a reserve source of energy available for use during fasting; it is an insulating material against cold and mechanical injuries.
It is also important that lipids, when decaying, release not only energy, but also a significant amount of water:
When 1 gram of protein is oxidized, 0.4 g is released; carbohydrates - 0.5 g; lipids - 1 g of water. This property of lipids is of great importance for animals living in the desert (camels).
Digestion of lipids in the gastrointestinal tract
In the oral cavity, lipids are only mechanically processed. There is a small amount of lipase in the stomach, which hydrolyzes fats. Low activity of lipase of gastric juice is associated with the acidic reaction of the contents of the stomach. In addition, lipase can only affect emulsified fats; there are no conditions in the stomach for the formation of fat emulsions. Only in children and monogastric animals does gastric acid lipase play an important role in lipid digestion.
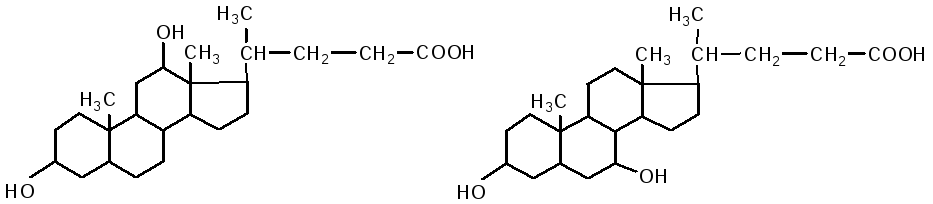
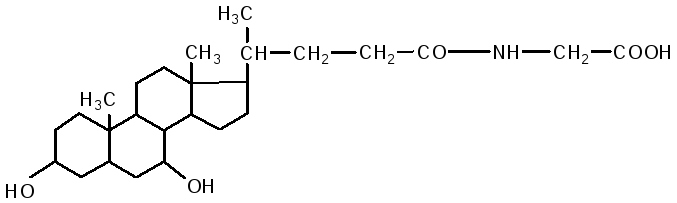
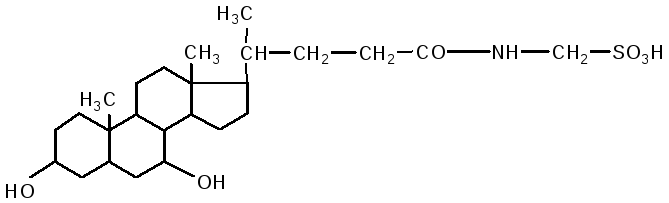
The intestine is the main site for the digestion of lipids. V duodenum lipids are affected by liver bile and pancreatic juice, while the intestinal contents (chyme) are neutralized. Fats are emulsified by bile acids. Bile contains: cholic acid, deoxycholic (3.12 dihydroxycholanic), chenodeoxycholic (3.7 dihydroxycholanic) acids, sodium salts of paired bile acids: glycocholic, glycodeoxycholic, taurocholic, taurodeoxycholic. They are composed of two components: cholic and deoxycholic acids, as well as glycine and taurine.
deoxycholic acid chenodeoxycholic acid
glycocholic acid
taurocholic acid
Bile salts emulsify fats well. This increases the area of contact of enzymes with fats and increases the effect of the enzyme. Insufficient synthesis of bile acids or delayed intake disrupts the effectiveness of enzymes. Fats are usually absorbed after hydrolysis, but some of the finely emulsified fats are absorbed through the intestinal wall and pass into the lymph without hydrolysis.
Esterases break the ester bond in fats between the alcohol group and the carboxyl group of carboxylic acids and inorganic acids (lipase, phosphatase).
Lipase hydrolyzes fats into glycerol and higher fatty acids. Lipase activity is increased by the action of bile, i.e. bile directly activates lipase. In addition, the activity of lipase is increased by Ca ++ ions due to the fact that Ca ++ ions form insoluble salts (soaps) with liberated fatty acids and prevent their suppressive effect on lipase activity.
Under the action of lipase, at the beginning, ether bonds are hydrolyzed at the α and α 1 (side) carbon atoms of glycerol, then at the β-carbon atom:
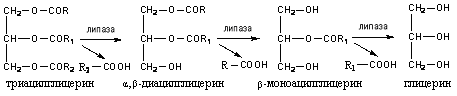
Under the action of lipase, up to 40% of triacylglycerides are broken down to glycerol and fatty acids, 50-55% is hydrolyzed to 2-monoacylglycerols and 3-10% is not hydrolyzed and absorbed as triacylglycerols.
The sterides of the feed are broken down by the enzyme cholesterol esterase to cholesterol and higher fatty acids. Phosphatides are hydrolyzed under the influence of phospholipases A, A 2, C and D. Each enzyme acts on a specific ester bond of the lipid. The points of application of phospholipases are shown in the diagram:
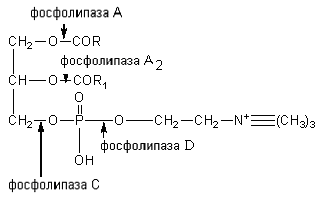
Phospholipases of the pancreas, tissue phospholipases are produced in the form of enzymes and are activated by trypsin. Phospholipase A 2 of snake venom catalyzes the elimination of unsaturated fatty acid at position 2 of phosphoglycerides. In this case, lysolecithins with hemolytic action are formed.
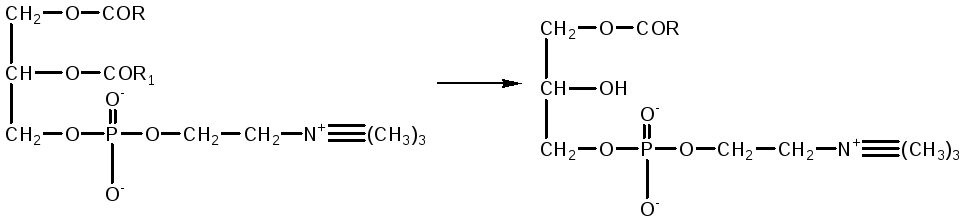
phosphotidylcholine lysolecithin
Therefore, when this poison enters the bloodstream, severe hemolysis occurs. In the intestine, this danger is eliminated by the action of phospholipase A1, which rapidly inactivates lysophosphatide as a result of the cleavage of a saturated fatty acid residue from it, converting it into inactive glycerophosphocholine.
Lysolecithins in low concentrations stimulate the differentiation of lymphoid cells, the activity of protein kinase C, and enhance cell proliferation.
Colamine phosphatides and serine phosphatides are cleaved by phospholipase A to lysocolamine phosphatides, lysoserine phosphatides, which are further cleaved by phospholipase A 2 . Phospholipases C and D hydrolyze choline bonds; colamine and serine with phosphoric acid and the remainder of phosphoric acid with glycerol.
Lipid absorption occurs in the small intestine. Fatty acids with a chain length of less than 10 carbon atoms are absorbed in unesterified form. For absorption, the presence of emulsifying substances is necessary - bile acids and bile.
Resynthesis of fat characteristic of a given organism occurs in the intestinal wall. The concentration of lipids in the blood is high within 3-5 hours after ingestion. Chylomicrons- small particles of fat, formed after absorption in the intestinal wall, are lipoproteins surrounded by phospholipids and a protein membrane, inside they contain molecules of fat and bile acids. They enter the liver, where lipids undergo intermediate metabolism, and bile acids pass to gall bladder and back into the intestines (see Figure 9.3 on page 192). As a result of this circulation, a small amount of bile acids is lost. It is believed that a molecule of bile acid makes 4 circuits per day.
Neutral fats include a group of lipids consisting of a triatomic alcohol - glycerol and three residues of fatty acids, therefore they are called triglycerides.
The composition of neutral fats can contain the same fatty acids, such as palmitic. In this case, an ester is formed - triglyceride, tripalmitin. These are simple fats. If the fats contain residues of different fatty acids, then mixed fats are formed.
This reaction equation shows the reversible processes of synthesis (upper arrow) and hydrolysis (lower) of fat.
Natural fats are distinguished by a wide variety of fatty acids included in their composition, their different arrangement in the molecule and the degree of unsaturation. There are potentially millions of triglyceride isomers.
Fatty acids are organic acids with a long hydrocarbon chain (radical R) containing from 4 to 24 or more carbon atoms and one carboxyl group. General formula fatty acids has the form
CnH2n + 1COOH, or R-COOH.
Many fatty acids are characterized by the presence of an even number of carbon atoms, which is apparently due to their synthesis by adding two-carbon units to the growing hydrocarbon chain.
Fatty acids with 16 or 18 carbon atoms, which are called higher fatty acids, are most often part of the fats of the human body. Higher fatty acids are divided into saturated saturated) and unsaturated (unsaturated)
In saturated fatty acids, all free bonds of carbon atoms are filled with hydrogen. Such fatty acids do not have double or triple bonds in the carbon chain. Unsaturated fatty acids have double bonds in the carbon chain (-C = C-), the first of which occurs between the ninth and tenth carbon atoms from the carboxyl group. Fatty acids with triple bonds are rare. Fatty acids containing two or more double bonds are called polyunsaturated.
With an increase in the number of carbon atoms in fatty acid molecules, their melting point increases. Fatty acids can be solids(for example, stearic) or liquid (for example, linoleic, arachidonic); they are insoluble in water and very slightly soluble in alcohol.
Solid fats are fats of animal origin, with the exception of fish oil... Liquid fats are vegetable oils except for coconut and palm oils, which solidify on cooling. In the body of animals and plants, unsaturated fatty acids are twice as large as saturated ones.
Unsaturated fatty acids are more reactive than saturated ones. They easily attach two hydrogen atoms at the site of double bonds, turning into saturated ones:
This process is called hydrogenation. Substances subjected to hydrogenation change their properties. For example, vegetable oils are converted to solid fat. The hydrogenation reaction is widely used to obtain solid edible fat - margarine from liquid vegetable oils.
Polyunsaturated fatty acids are of particular importance to humans. They are not synthesized in the body. With their lack or absence in food, the metabolism of fats, in particular cholesterol, is disturbed, pathological changes are observed in the liver, skin, and platelet function. Therefore, unsaturated fatty acids such as linolenic and linoleic are essential nutritional factors.
In addition, they promote the release of fats from the liver, which are synthesized in it, and prevent obesity. This action of unsaturated fatty acids is called the lipotropic effect. Unsaturated fatty acids serve as precursors for the synthesis of biologically active substances- prostaglandins. The daily requirement of a person for polyunsaturated acids is normally about 15 g.
Neutral fats accumulate in fat cells (adipocytes), under the skin, in the mammary glands, fat capsules around internal organs abdominal cavity; a small amount is found in skeletal muscles. The formation and accumulation of neutral fats in adipose tissues is called deposition. Triglycerides form the basis of reserve fats, which are the body's energy reserve and are used during fasting, insufficient intake of fats, prolonged physical activity.
Neutral fats are also found in cell membranes, complex proteins protoplasm and are called protoplasmic. Protoplasmic fats are not used as an energy source even when the body is depleted, since they perform a structural function. Their number and chemical composition are constant and do not depend on the composition of food, while the composition of reserve fats is constantly changing. In humans, protoplasmic fats account for about 25% of the total body fat mass (2-3 kg).
In various cells of the body, especially in adipose tissue, enzymatic reactions of biosynthesis and disintegration of neutral fats constantly occur:
During the hydrolysis of fats in the body, glycerol and free fatty acids are formed. This process is catalyzed by lipase enzymes. The process of hydrolysis of fats in the tissues of the body is called lipolysis. The rate of lipolysis increases significantly with physical endurance exercise, and the activity of lipases increases during training.
If the fat decomposition reaction is carried out in the presence of alkalis (NaOH, KOH), then sodium or potassium salts of fatty acids are formed, which are called soaps, and the reaction itself is saponification. This chemical reaction underlies the production of soap from various fats and mixtures thereof.
Phospholipids
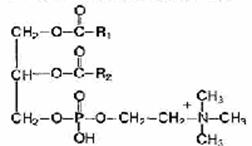
Phospholipids are fat-like substances consisting of alcohol (usually glycerol), two residues of fatty acids, a phosphoric acid residue and a nitrogen-containing substance (amino alcohol - choline or colamine).
If choline is included in the phospholipid molecules, they are called lecithins, and if colamine - cephalins.
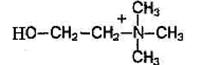
 Choline Colamine
Choline Colamine
Alpha Lecithin Alpha Cephalin
The structure of beta isomers differs in that the residues of phosphoric acid and amino alcohol are located at the second (middle) carbon atom of glycerol.
Phosphatides, especially lecithin, are found in large quantities in the yolk of eggs. In the human body, they are widely distributed in the nervous tissue. Phospholipids play an important biological role, being a structural component of all cell membranes, suppliers of choline, which is necessary for the formation of a neurotransmitter, acetylcholine. Phospholipids depend on such membrane properties as permeability, receptor function, catalytic activity of membrane-bound enzymes.
Phospholipids dominate membranes animal cell, they are also contained in many of its subcellular particles.
The biological role of phospholipids in the body is significant and varied. As an indispensable component biological membranes fofolipids take part in their barrier, transport, receptor functions, in the division of the inner space of the cell into cellular organelles - "cisterns", compartments. These functions of membranes are currently considered to be the most important regulatory mechanisms of cell activity. The presence of phospholipids in membranes is also necessary for the functioning of membrane-bound enzyme systems.
STEROIDS
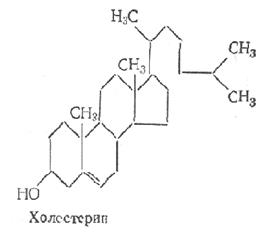
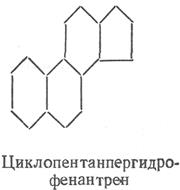
Steroids are classified as unsaponifiable lipids. By their chemical nature, steroids are derivatives of cyclopentaneperhydrofenanthrene. They are divided into sterols and sterols. Sterols are high molecular weight cyclic alcohols with a nucleus in the molecule.
 |
Various tissues also contain sterides - esters formed by sterols and fatty acids. Sterols and their derivatives perform various functions in the body. Big biological significance in the animal body has cholesterol. Violation of his exchange may entail pathological changes vessels - atherosclerosis. Cholesterol serves as a biological precursor of bile acids, steroid hormones. Bile acids have great importance in the process of lipid breakdown in the intestine. Steroid hormones regulate numerous metabolic processes.
PROTEINS
The most important compounds in every organism are proteins. They are necessarily found in all cells of the body; in most of them, protein accounts for more than half of the dry residue. All major manifestations of life are associated with proteins. “Life,” wrote F. Engels, “is a way of existence of protein bodies ... Wherever we meet life, we find that it is connected with some protein body, and everywhere we meet some protein body, that is not in the process of decay, we, without exception, meet the manifestations of life. "
Proteins - high-molecular nitrogen-containing organic compounds consisting of amino acid residues. In the composition of some proteins, along with amino acids, other compounds are also found.
Living organisms are characterized by a wide variety of proteins that form the basis of the structure of the body and provide many of its functions. It is believed that there are approximately 1010-1012 different proteins in nature, which explains the large variety of living organisms. In unicellular organisms, there are about 3,000 different proteins, and in the human body - about 5,000,000.
Despite the complexity of their structure and diversity, all proteins are built from relatively simple structural elements - amino acids. Proteins are polymeric molecules containing 20 different amino acids. A change in the number of amino acid residues and the sequence of their arrangement in a protein molecule provides the possibility of the formation of a huge amount of proteins that differ in their physicochemical properties, structural or functional role in the body.
For any organism, proteins play a decisive role in all life processes. Associated with them are such properties of a living organism as irritability, contractility, digestion, the ability to grow, reproduce, and move. Consequently, squirrels are the main carriers of life. In inanimate nature, compounds like proteins are not found.
Chemical composition and biological role of proteins
Proteins are high-molecular nitrogen-containing substances, the hydrolysis of which produces amino acids. Sometimes proteins are called proteins (from the Greek proteus - the first, main), thus determining their most important role in the life of all organisms. Protein in the human body accounts for an average of 45% of dry body weight (12-14 kg). Its content in individual tissues is different. The largest amount of protein is found in muscles, bones, skin, digestive tract and other dense tissues.
The daily protein requirement of an adult who is not involved in sports is on average 1.3 g per 1 kg of body weight, or about 80 g. With high energy consumption, the need for them increases by about 10 g for every 2100 kJ of increasing energy consumption ...
Proteins enter the body mainly with food of animal origin. Plants contain significantly less proteins: in vegetables and fruits - only 0.3-2.0% of the mass of fresh tissue; the largest amount of proteins - in legumes - 20-30%, cereals - 10-13 and mushrooms - 3-6%.
Elemental composition of proteins. The most important chemical elements of all proteins are carbon (50-55%), oxygen (21-23%), hydrogen (6.5-7.3%), nitrogen (15-18%), sulfur (0.3- 2.5%). The proteins also contain phosphorus, iron, iodine, copper, manganese and other chemical elements.
Lipids are simple and complex. Simple ones consist of two components (for example, neutral fats contain glycerin and fatty acids), and complex fats have more than two.
Simple lipids include fats (triglycerols or neutral fats) and waxes. Their essential component is fatty acids.
Fatty acids (FA) are monocarboxylic acids with one aliphatic chain, i.e. consisting of one carboxyl group and a long non-polar tail.
Fatty acids of natural lipids usually contain an even number of carbon atoms
Fatty acids are subdivided into saturated (or saturated) and unsaturated (unsaturated). Saturated acids do not contain double bonds. Unsaturated acids contain one (monounsaturated) or several (polyunsaturated) double bonds:
CH 3 (CH 2) nCH = CH (CH 2) nCOOH - monounsaturated;
CH 3 (CH 2) n (CH = CHCH 2) m (CH 2) kСООН - polyunsaturated
Double bonds in natural polyunsaturated fatty acids are isolated (non-conjugated). As a rule, the bonds have a cis-configuration, which gives these molecules additional rigidity. This makes biological sense, because such molecules are part of cell membranes.
Let's give their classification.
Of unsaturated fatty acids, palmitic and stearic ones are most often found.
C 16: 0 - the abbreviation for palmitic acid - means that it has 16 carbon atoms and no double bonds.
CH 3 (CH 2) 14 COOH is another designation for palmitic acid
C 18: 0 - stearic, CH 3 (CH 2) 16 COOH
In addition, the following saturated fatty acids are secreted:
C 12: 0 - lauric;
C 14: 0 - myristic;
C 20: 0 - arachidic;
C 22: 0 - behenic;
C 24: 0 - lignoceric acid.
Monoenes:
C 16: 1 - palmitooleic
CH 3 (CH 2) 5 CH = CH (CH 2) 7 COOH;
C 18: 1 - oleic
CH 3 (CH 2) 7 CH = CH (CH 2) 7 COOH.
The position of the double bond relative to the carboxyl group is denoted by the sign ∆ 9, where the number indicates the serial number of the carbon atom near which the double bond is located. Thus, the named acids can be designated respectively C 16: 1, ∆ 9 and C 18: 1, ∆ 9.
Polyenoic acids most often they are with two and three double bonds:
C 18: 2, ∆ 9 - linoleic, CH 3 (CH 2) 4 (CH = CHCH 2) 2 (CH 2) 6 COOH;
C 18: 3, ∆ 9 - linolenic, CH 3 CH 2 (CH = CHCH 2) 3 (CH 2) 6 COOH.
Sometimes fatty acids (so-called unusual) are found, in the aliphatic chains of which there are substituents: CH 3 -, -OH, C = O, etc.:
CH 3 (CH 2) 7 -CH- (CH 2) 8 COOH - tuberculostearin, C 19: 0, from tuberculosis sticks
CH 3 (CH 2) 5 -CH - CH (CH 2) 9 COOH - lactobacillus C 19: 0.
Fatty acids are insoluble in water, the melting point decreases with an increase in the number of double bonds and shortening of the chain.
Fatty acids such as linoleic, linolenic and the like (with two and three double bonds) are not synthesized inside the human body and are called essential. Therefore, they must be obtained from food.
In this case, polyenoic acids are divided into two groups: ω-3 and ω-6 (depending on the position of the double bond from the carbon atom of the last, methyl group). These acids are precursors of different groups of topical hormones - eicosanoids. Thus, linoleic acid is an example of ω-6 acids. An example of ω-3 acids is timnodonic (eicosapentanoic) acid, C 20: 5 (ω-3). It is found in the fat of marine fish, although it is of plant origin, it is synthesized by phytoplankton. In addition, fish such as salmon, mackerel, herring, sardines, etc., eating plankton, accumulate this acid in their fat. When a person eats this acid, his blood clotting decreases, which is used to prevent cardiovascular diseases.
Waxes
Waxes are esters formed by long chain fatty acids and long chain alcohols (16 to 36 carbon atoms). Waxes are widespread in nature. Wax coating of leaves and fruits of plants protects them from mechanical damage, reduces moisture loss, and prevents infection. In vertebrates, waxes secreted by the skin glands act as a protective coating that lubricates and softens the skin and protects it from water. The hair is covered with a wax secret. Bird feathers and animal skins are also wax-coated, which makes them water-repellent. Wax sheep wool- lanolin - is widely used in medicine and cosmetics as a basis for the preparation of ointments and creams. The wax produced by bees serves as the building material of the honeycomb:
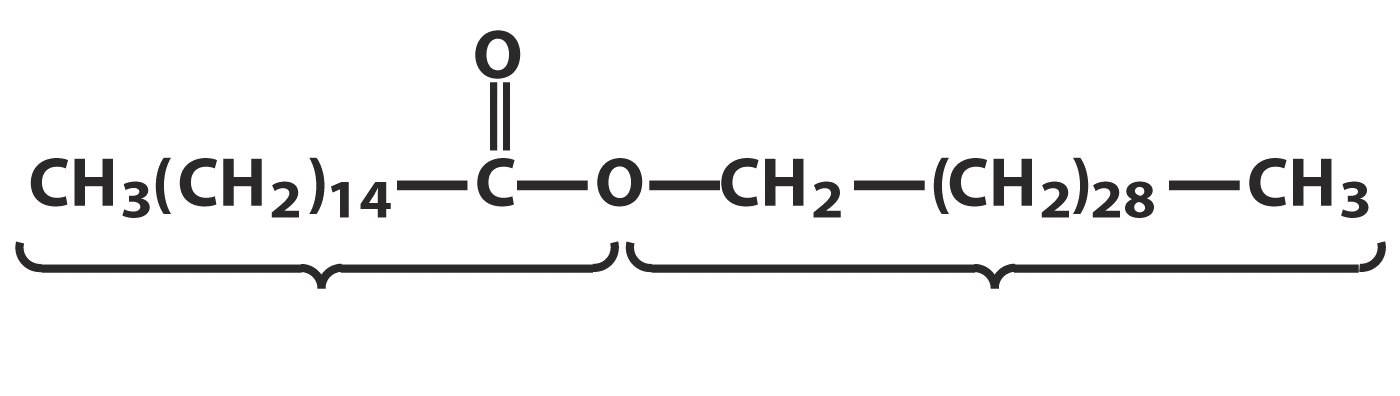
Beeswax
Waxes are normal metabolites of some microorganisms. Natural waxes, along with esters of higher fatty acids and higher alcohols, contain a certain amount of free fatty acids, alcohols, as well as hydrocarbons with an odd number of carbon atoms (21-35), dyes and fragrances. All waxes are solids of various colors, resistant to light, oxidants, and heat. Their melting point is from 30 to 90 o C.
Neutral fats (triacylglycerols, triglycerides)
These are esters of glycerol and fatty acids. Neutral fats are available in simple and blended forms. Simple ones contain the same residues of fatty acids, mixed ones - residues of different fatty acids. Neutral fats can contain both saturated and unsaturated fatty acids.
Neutral fats are divided into triacylglycerides, diacylglycerides, and monoacylglycerides (depending on the amount of fatty acids attached to glycerol). The most common are triacylglycerides. The names of triacylglycerols are derived from the names of the fatty acids that make up their composition. For example, triacylglycerol containing three palmitic acid residues would be called tripalmitin:
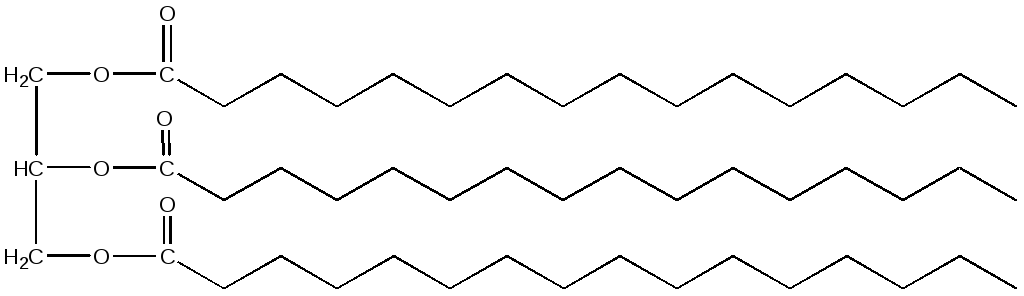
If the molecule contains residues of various fatty acids, then the name will contain all the residues included in its composition with the ending -oyl and the addition of the word glycerol. For example, 1-stearoyl, 2-linoleoyl, 3-palmitoyl glycerol:
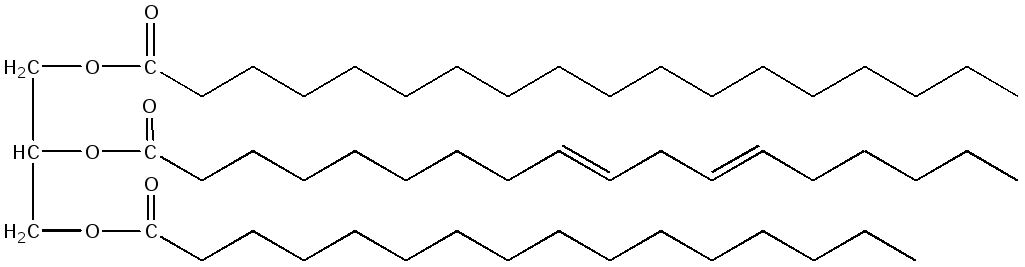
Physicochemical properties of triglycerides are determined by the properties of their constituent fatty acids. As a rule, animal triacylglycerides contain more saturated acids than vegetable ones and are therefore harder. The composition and quality of fat are characterized by special parameters called chemical triglyceride constants:
1) iodine number is the number of grams of iodine that is bound by 100 grams of fat. Since iodine binds only to double bonds of fatty acids, the iodine number characterizes the degree of fat unsaturation.
2) acid number - the number of milligrams of potassium hydroxide required to neutralize 1 gram of fat. Indicates the amount of free fatty acids in the fat.
3) the number of saponification - the number of milligrams of potassium hydroxide required to neutralize all fatty acids, free and bound, that make up the fat.
PHYSICO-CHEMICAL PROPERTIES OF LIPIDS
Lipids are very heterogeneous in their chemical structure, substances characterized by different solubility in organic solvents and, as a rule, insoluble in water. They play an important role in life processes. As one of the main components of biological membranes, lipids affect their permeability, are involved in the transmission of nerve impulses, and the creation of intercellular contacts.
Other functions of lipids are the formation of an energy reserve, the creation of protective water-repellent and thermal insulating covers in animals and plants, the protection of organs and tissues from mechanical stress. The lipid fraction contains the majority of substances, of which are presented in the table. Due to the heterogeneity of the components included in the lipid fraction, the term "lipid fraction" cannot be regarded as a structural characteristic; it is only a working laboratory name for the fraction obtained from the extraction of biological material with non-polar solvents. However, most lipids share some common structural features that give rise to important biological properties and similar solubility. The most common lipids are neutral fats, the structural component of which, like most lipids, is fatty acids.
SOME NATURAL FATTY ACIDS
|
Number of carbon atoms |
Structure |
Systematic name |
Trivial name |
|
Saturated fatty acids |
|||
|
CH 3 (CH 2) 10 COOH |
n- Dodecane |
Lauric |
|
|
CH 3 (CH 2) 12 COOH |
n- Tetradecane |
Myristic |
|
|
CH 3 (CH 2) 14 COOH |
n- Hexadecanoic |
Palmitic |
|
|
CH 3 (CH 2) 16 COOH |
n- Octadecane |
Stearic |
|
|
CH 3 (CH 2) 18 COOH |
n- Eicosane |
Arachinic |
|
|
CH 3 (CH 2) 22 COOH |
n- Tetracosan |
Lignoceric |
|
|
Unsaturated fatty acids |
|||
|
CH 3 (CH 2) 5 CH = CH (CH 2) 7 COOH |
Palmitoleic |
||
|
CH 3 (CH 2) 7 CH = CH (CH 2) 7 COOH |
Oleinovaya |
||
|
CH 3 (CH 2) 4 CH = CHCH 2 CH = CH (CH 2) 7 COOH |
Linoleic |
||
|
CH 3 CH 2 CH = CHCH 2 CH = CHCH 2 CH = CH (CH 2) 7 COOH |
Linolenic |
||
|
CH 3 (CH 2) 4 CH = CHCH 2 CH = CHCH 2 CH = CHCH 2 CH = (CH 2) 3 COOH |
Arachidonic |
||
Fatty acids - aliphatic carboxylic acids - in the body can be in a free state (trace amounts in cells and tissues) or serve as building blocks for most classes of lipids.
Natural fatty acids, although somewhat conditionally, can be divided into three groups: saturated, monounsaturated and polyunsaturated fatty acids. Fatty acids found in natural lipids generally contain an even number of carbon atoms and are predominantly unbranched.
The fatty acids that make up the lipids of animals and higher plants have many properties in common. As already noted, almost all natural fatty acids contain an even number of carbon atoms, most often 16 or 18. The unsaturated fatty acids of animals and humans, which are involved in the construction of lipids, usually contain a double bond between the 9th and 10th carbon atoms; additional double bonds, as a rule, are located between the 10th carbon atom and the methyl end of the chain. The peculiarity of double bonds of natural unsaturated fatty acids lies in the fact that they are always separated by two simple bonds, that is, there is always at least one methylene group between them (-CH = CH - CH 2 - CH = CH -). Such double bonds are referred to as "isolated". Natural unsaturated fatty acids have cis-configuration and extremely rare trance-configurations. It is believed that in unsaturated fatty acids with multiple double bonds cis- the configuration gives the hydrocarbon chain a curved and shortened appearance, which makes biological sense (especially considering that many lipids are part of membranes).
Long chain fatty acids are practically insoluble in water. Their sodium and potassium salts (soaps) form micelles in water. In the latter, negatively charged carboxyl groups of fatty acids face the aqueous phase, and non-polar hydrocarbon chains are hidden inside the micellar structure. Such micelles have a net negative charge and remain suspended in solution due to mutual repulsion.
THEME. 1.PHYSICOCHEMICAL PROPERTIESNEUTRAL FATS
(8 ocloc'k)
Neutral fats are esters of glycerol and fatty acids. If all three hydroxyl groups of glycerol are esterified with fatty acids (acyl radicals R 1, R 2 and R 3 may be the same or different), then such a compound is called triglyceride
Glycerin (glycerol) Monoglyceride (monoacylglycerol)
(triacylglycerol), if two - with diglyceride (diacylglycerol) and, finally, if one group is etirified - with monoglyceride (mono-acylglycerol).
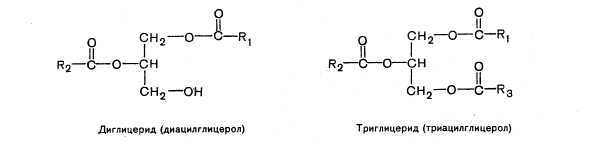
Diglyceride (diacylglycerol) Triglyceride (triacylglycerol)
Neutral fats are found in the body either in the form of protoplasmic fat, which is a structural component of cells, or in the form of reserve, reserve fat. The role of these two forms of fat in the body is not the same. Protoplasmic fat has a constant chemical composition and is contained in tissues in a certain amount, which does not change even with morbid obesity, while the amount of reserve fat is subject to large fluctuations.
The bulk of natural neutral fats are triglycerides. The fatty acids in triglycerides can be saturated or unsaturated. Palmitic, stearic and oleic acids are more common among fatty acids. If all three acid radicals belong to the same fatty acid, then such triglycerides are called simple (for example, tripalmitin, tristearin, triolein, etc.), but if they are different fatty acids, then they are called mixed. Mixed triglycerides are named from their constituent fatty acids; the numbers 1, 2 and 3 indicate the bond of the fatty acid residue with the corresponding alcohol group in the glycerol molecule (for example, 1-oleo-2-palmitostearin).
Fatty acids that make up triglycerides practically determine their physicochemical properties... Thus, the melting point of triglycerides increases with an increase in the number and the length of the residues of saturated fatty acids . In contrast, the higher the content of unsaturated fatty acids or short-chain acids, the lower the melting point. Animal fats (lard) usually contain a significant amount of saturated fatty acids (palmitic, stearic, etc.), due to which they are solid at room temperature. Fats, which contain many mono- and polyunsaturated acids, are liquid at ordinary temperatures and are called oils. Thus, in hemp oil, 95% of all fatty acids are oleic, linoleic and linolenic acids, and only 5% are stearic and palmitic acids. Note that human fat melting at 15 ° C (at body temperature it is liquid) contains 70% oleic acid.
Glycerides are able to enter into all chemical reactions inherent in esters. Highest value has a saponification reaction, as a result of which glycerol and fatty acids are formed from triglycerides. Saponification of fat can occur both during enzymatic hydrolysis and under the action of acids or alkalis.
Laboratory work No. 40
EDUCATIONOIL SPOT
Progress
A drop of oil is applied with a glass stick to a piece of paper. A stain is formed that does not disappear when heated.
Laboratory work No. 41
FAT SOLUBILITY
Reagents: Vegetable oil (sunflower, linseed, cottonseed or other)
Solid fat (mutton, beef)
Diethyl ether, acetone
Ethanol
Progress
Place two rows of tubes, 4 in each. A few drops of vegetable oil are introduced into the test tubes of the first row, a piece of solid fat into the test tubes of the second row. 2 ml of distilled water is poured into the first test tube of each row, the same amount of diethyl ether is poured into the second, the same amount of acetone, and the fourth alcohol. All tubes are shaken and the solubility of fats in various solvents is observed. It is recommended to warm up tubes with alcohol in a water bath. The results of the experiment are recorded.
|
Experience Option |
test tubes |
Reagents used (ml) |
Solubility degree |
|||
|
Vegetable oil |
||||||
Make a conclusion:
Laboratory work No. 42
EMULSION OF FATTY OILS
Reaktives: Vegetable oil
Sodium carbonate, 2% solution
Soap, 2% solution
Progress
5 drops of oil are added to four test tubes. In the first tube add 2 ml of distilled water, in the second - 2 ml of 2% sodium carbonate (soda) solution, in the third - the same 2% soap solution, in the fourth - 2 ml of water and a few drops of bile. All test tubes are shaken and observe the formation in the first test tube of an unstable emulsion of oil in water, which quickly exfoliates upon standing, and in the rest - a stable emulsion due to the action of added emulsifiers, which are adsorbed in the outer layer of fat droplets and lower their surface tension.
Enter the result of the experiment into the table:
|
test tubes |
Reagents used (ml) |
The nature of the emulsion |
||||
|
Vegetable |
H 2 O + bile |
|||||
|
A also students medical universities Of Ukraine. Methodical directions drawn up Assoc.... Fedorko N.L., Assoc.... Zakharieva Z.E., Assoc.... Vovchuk ... Safety in emergencies analysis of the provision of prehospital medical care to victims of road traffic accidents with concomitant injuries in the arctic zone of the arkhangelsk regionDocumentDoctor, students humanitarian and medical specialties highlighted that the doctor should to be highly qualified specialist, use best practices ... L. N. Viktorova Candidate of Law Sciences, Assoc.Prof. Ch. 21 (et al.)Document... on checking pharmacies and others medical institutions, for people engaged in crafts medical recipes ... maybe to be the time of the onset of such consequences is fixed, which found out during the investigative examination, with the help specialist. These ... L. S. Volkova and Honored Worker of the Higher School of the Russian Federation, Professor (1)DocumentSo what on damage to both another produces aphasia, only, maybe to be, form, in which put it ... and medical institutions... Due to the fact that students departments of oligophrenopedagogy are received during training in university also and speech therapy ... I. A. Altman (editor-in-chief), A. E. Gerber, Yu. A. Dombrovsky, Yu. I. Kanner, B. N. Kovalev, G. V. Kostyrchenko, Dr. Tamás Kraus (Hungary), A I. Kruglov (Ukraine), D. I. Poltorak, E. S. Rosenblat (Belarus), L. ADocumentFor specialists educational systems - leaders, teachers, psychologists, social educators, medical educational workers institutions, a also ... | ||||||
Acylglycerols, or neutral lipids - the most common group of lipids in nature. These compounds are esters of fatty acids and a trihydric alcohol of glycerol (glycerides), in which one, two or three hydroxyl groups of glycerol can be esterified to form, respectively mono-, di- and triacylglycerols:
Triacylglycerols are most commonly found in nature. Since all of the above acylglycerols do not contain ionic groups, they belong to neutral lipids. If all three acid radicals belong to the same fatty acid, then such triacylglycerols are called simple, if they are different fatty acids, then they are called mixed.
The fatty acids that make up the triacylglycerols determine their physicochemical properties. The more residues of short-chain and unsaturated acids in lipids, the lower the melting point and the higher the solubility. For example, animal fats usually contain a significant amount of saturated fatty acids, due to which they remain solid at room temperature. Fats, which contain many unsaturated acids, will be liquid under these conditions; they are called oils.
Most animal fats contain in various proportions esters of palmitic, stearic, palmitooleic, oleic and linoleic acids. Human fat melting at 15 ° C contains about 70% unsaturated fatty acids, and at body temperature it is in a liquid state. Fats from different tissues of one organism, like vegetable oils, can differ from each other both in the length of hydrocarbon chains and in the degree of their unsaturation.
Constants are used to characterize the properties of fat, or fat numbers- acid number, saponification number, iodine number.
The common structural fragment of all phosphoglycerides is phosphatidic acid (1,2-diacyl, 3-phosphoglycerol).
Phosphatidic acid is formed in the body during the biosynthesis of triacylgl and nerols and phosphoglycerides as a common intermediate metabolite; in tissues, it is present in small quantities. It should be noted that all natural phosphoglycerides belong to the L-series. Various phosphoglycerides differ from each other by additional groups attached by a phosphoester bond to phosphatidic acid, i.e. R3. The fatty acid composition of various phosphoglycerides differs even within the same organism and, along with substituent groups, determines the specificity of phospholipids:
Phosphatidylcholine (lecithin). It contains the amino alcohol choline (3-hydroxyethyltrimethylammonium hydroxide):
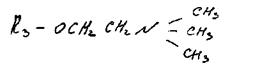 |
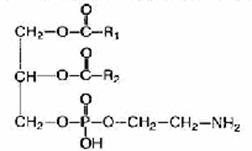
Phosphatidylethanolamine (cephalin). Instead of choline, the composition of phosphatidylethanolamines contains the nitrogenous base ethanolamine HO-CH 2 -CH 2 -NH 3.
In the body of animals and in higher plants in the greatest number there are phosphatidylcholines and phosphatidylethanolamines. These two groups of glycerophospholipids are the main lipid components of cell membranes.
Phosphatidylinositols Unlike other groups of phosphoglycerides, the composition of phosphatidylinositols instead of nitrogen-containing compounds includes the 6-carbon cyclic alcohol inositol, represented by one of its stereoisomers, monositol.
Phosphatidylglycerols. Like phosphatidylinositols, phosphatidylglycerols do not contain nitrogen-containing compounds. In these compounds, another glycerol molecule serves as a polar group.


