Vitamin D - Calciferol, ergosterol, viosterol
We acquire it through sunlight or through food. Ultraviolet rays act on skin oils, promoting the formation of this vitamin, which is then absorbed into the body. Vitamin D is formed in the skin from provitamins under the influence of sunlight. Provitamins, in turn, partially enter the body in finished form from plants (ergosterol, stigmasterol and sitosterol), and are partially formed in the tissues of their cholesterol (7-dehydrocholesterol (provitamin D3).
In addition, sex hormones, prolactin, growth hormone, and insulin-like growth factor 1 increase renal production of the active metabolite. On the contrary, hypercalcemia reduces vitamin synthesis. In addition, the decrease in phosphatemia increases, and its increase reduces the production of the active vitamin. The resulting value indicates whether the inhalation is sufficient or insufficient. For this reason, it is currently considered that deposits of this vitamin are sufficient if their plasma concentration is above this value4.
Nutritional requirements are defined as the amount of each nutrient that an individual needs for optimal health. They vary depending on age, gender and physiological characteristics such as pregnancy and lactation.
When taken orally, vitamin D is absorbed from fats through the walls of the stomach.
Measured in International Units (IU). The daily dose for adults is 400 IU or 5-10 mcg. After tanning, the production of vitamin D through the skin stops.
Benefit: Properly utilizes calcium and phosphorus, necessary for strengthening bones and teeth. When taken together with vitamins A and C, it helps in the prevention of colds. Helps in the treatment of conjunctivitis.
Although the recommended intake refers to the average daily intake a person should achieve, the amount ingested may vary from day to day. In general, intake is assessed over a period of 7-15 days, so that over time the amount consumed matches the recommendations.
When does the need for vitamin D increase?
In addition, maximum safe intakes or maximum tolerable intakes have been established for some nutrients. This refers to the level of consumption above which there may be a health risk. Long-term or widespread exposure has not shown excess production of cholecalciferol at levels capable of causing toxicity.
Diseases caused by vitamin D deficiency: rickets, severe tooth decay, osteomalacia*, senile osteoporosis.
Vitamin D belongs to the group of fat-soluble vitamins with antirachitic effects (D 1, D 2, D 3, D 4, D 5)
Vitamins of group D include:
vitamin D 2 - ergocalciferol; isolated from yeast, its provitamin is ergosterol; vitamin D 3 - cholecalciferol; isolated from animal tissues, its provitamin is 7-dehydrocholesterol; vitamin D 4 - 22, 23-dihydro-ergocalciferol; vitamin D 5 - 24-ethylcholecalciferol (sitocalciferol); isolated from wheat oils; itamin D 6 - 22-dihydroethylcalciferol (stigma-calciferol).
Firstly, it depends on the amount of 7-dehydrocholesterol in the epidermis. Older people have lower amounts of 7-dehydrocholesterol in their skin. Secondly, it depends on the amount of melanin. People with higher melanin content require longer sun exposure to synthesize the same amount of cholecalciferol. This is due to the fact that melanin absorbs photons from the sun. Likewise, sunscreen creams absorb radiation before penetrating the skin. Creams with a protection factor of 15 reduce their capacity by more than 98%.
in winter Sun rays penetrate the ground at a more inclined angle. In this situation, more photons are absorbed by the ozone layer. Likewise, in the early hours of the morning and late afternoon, the angle at which the sun's rays penetrate the ground is more slanted. At latitudes greater than 37° north and south of the equator, especially during the winter months, the number of photons reaching earth's surface, below. However, it is known that if sun exposure causes slight erythema, and immediately after applying sunscreen, the risk to the skin is minimal.
Today, vitamin D refers to two vitamins - D 2 and D 3 - ergocalciferol and cholecalciferol - these are colorless and odorless crystals that are resistant to high temperatures. These vitamins are fat soluble, i.e. dissolves in fats and organic compounds and are insoluble in water.
They regulate the metabolism of calcium and phosphorus: they participate in the process of calcium absorption in the intestine, interact with parathyroid hormone, and are responsible for bone calcification. In childhood, with vitamin D deficiency, due to a decrease in the content of calcium and phosphorus salts in the bones, the process of bone formation (growth and ossification) is disrupted, and rickets develops . In adults, bone decalcification occurs (osteomalacia).
Marine fish fatty acids are richest source colecalciferol, with salmon being the main source as it is the most commonly consumed. Eggs, butter, liver and others internal organs are also foods that contain the vitamin, but its intake is low due to its high content cholesterol. Table 3 shows the cholecalciferol content of the food groups.
Juices, milk and other dairy products are fortified with calcium. Food industry uses both ergocalciferol and colecalciferol for enrichment food products, although the latter are used more often. Vitamin content is not expressed in micrograms or international units, but as a percentage of adequate intake for adults 11.
The German chemist A. Windaus, who studied sterols for more than 30 years, in 1928 discovered ergosterol - provitamin D, which was converted into ergocalciferol under the influence of ultraviolet rays. It was found that under the influence of ultraviolet rays, a certain amount of vitamin D can be formed in the skin, and irradiation can be as solar and using a quartz lamp. . It is estimated that 10-minute irradiation of animals has the same effect on the body as introducing 21% into the diet fish oil. In irradiated foods, vitamin D is formed from special fat-like substances (sterols). IN Lately In animal husbandry, ultraviolet irradiation of animals, especially young animals, and feed is widely used.
Some dairy products, especially skim and semi-mixed milk, some juices and cereals are fortified different amounts vitamin A. Although food fortification has been shown to be useful in increasing population intake of this vitamin as is currently done, it has some disadvantages. On the one hand, milk is mainly fortified. However, milk is not a food consumed uniformly by the entire population, especially consumption by high-risk individuals such as the African American race and vegetarians.
Besides, in last years There is a downward trend in milk consumption among the population. This deficiency can be overcome by fortifying all dairy products, including yoghurts, cheeses and other dairy products. Other presentations containing a vitamin associated with other vitamins and minerals are not listed because there are many preparations. It should be remembered that in persons with chronic liver disease there is a significant change in the activity of liver 25-hydroxylase, so it is better to administer calcifediol instead of cholecalciferol.
Main sources: fish oil, caviar, liver and meat, egg yolk, animal fats and oils, sardines, herring, salmon, tuna, milk. hay flour, Vitamin D is found in large quantities and in egg yolk, yeast, good hay, vegetable oil, grass meal and other products. Plants, as a rule, do not contain the vitamin, but they contain the provitamin ergosterol, which is converted into vitamin D in the body of animals.
The adverse effects of these drugs are insufficient. At long-term treatment and at high doses, hypercalcemia may occur, which is avoided by adjusting the dose in accordance with oral dosage recommendations for the general population and corrected by stopping the drug. Consumption depends not only on age, but also on gender, race and the fact of special diets.
By age, population from greatest risk deficiency is the elderly. With age, 7-dehydrocholesterol levels in the skin decrease, sun exposure is lower, and the skin's ability to synthesize colecalciferol is 25% lower. However, intake of vitamin and mineral supplements is higher at this age, at least in the American population14. However, women over 50 years of age are more likely to consume nutrients, enriched with vitamin 15. The reason is twofold: on the one hand, the greater amount of melanin in the skin partially absorbs ultraviolet rays, reducing cutaneous synthesis of the vitamin, and on the other hand, lactose intolerance is more common in this group of individuals, so milk consumption is lower.
Daily requirement 2.5 mcg, for children and pregnant women - 10 mcg. Intestinal and liver disorders and gall bladder dysfunction negatively affect the absorption of vitamin D.
In pregnant and lactating animals, the need for vitamin D increases, because Additional amounts are needed to prevent rickets in children.
Action
Vitamin D is found in foods
Finally, strict vegetarian individuals tend to have low hormonal deposits. These percentages are lower than other nationalities17. This value is found in approximately 50% of patients who are hospitalized with an acute hip fracture. However, its presence has been found in almost all cells of the body, including the brain, heart, skin, pancreatic beta cells, gonads, prostate, breast, colon and immune system. As a result, the production and maturation of osteoclasts and the release of of hydrochloric acid and collagenases.
The main function of vitamin D is to ensure normal growth and development of bones, prevent rickets and osteoporosis. It regulates mineral metabolism and promotes calcium deposition in bone tissue and dentin, thus preventing osteomalacia (softening) of bones.
Upon entering the body, vitamin D is absorbed in the proximal small intestine, and always in the presence of bile. Part of it is absorbed in the middle sections small intestine, a small part - in the ileum. After absorption, calciferol is found in the composition of chylomicrons in free form and only partially in the form of ester. Bioavailability is 60-90%.
All this leads to the mobilization of calcium from bone deposits, so the concentration of calcium in the plasma is normal. In addition, secondary hyperparathyroidism induces loss of phosphorus in the urine, reducing plasma electrolyte levels. The result is an inadequate calcium-phosphorus product that contributes to defective bone matrix mineralization. Growth retardation, bone deformation, especially long bones, and increased risk fracture23. In an adult with the greatest presence of mineralized bone, bone deformities do not occur.
Vitamin D affects general exchange substances in the metabolism of Ca2+ and phosphate (HPO2-4). First of all, it stimulates the absorption of calcium, phosphates and magnesium from the intestines. An important effect of the vitamin in this process is to increase the permeability of the intestinal epithelium to Ca2+ and P.
Vitamin D is unique - it is the only vitamin that acts as both a vitamin and a hormone. As a vitamin, it maintains the levels of inorganic P and Ca in the blood plasma above the threshold value and increases the absorption of Ca in the small intestine.
However, osteoid does not mineralize properly, resulting in osteomalacia. As with osteoporosis, patients with osteomalasia have low mineral density bone tissue, measured by dual energy X-ray densitometry, and increased risk of fracture. Unlike osteoporosis, osteomalacia causes generalized bone pain and proximal muscle weakness. Sometimes, due to the presence of pain, the disease is confused with myositis, fibromyalgia or chronic fatigue syndrome24.
In addition, there is a study that shows a reduction in the risk of falls in older adults35. Today we know that the vitamin has many functions not only at the bone level, but also in many places in the body. However, fortifying milk as is done today is not enough. It is formed on the skin with exposure to ultraviolet rays in sufficient quantities to cover daily needs.
The active metabolite of vitamin D, 1,25-dioxycholecaciferol, which is formed in the kidneys, acts as a hormone. It affects the cells of the intestines, kidneys and muscles: in the intestines it stimulates the production of a carrier protein necessary for the transport of calcium, and in the kidneys and muscles it enhances the reabsorption of Ca++.
Vitamin D 3 affects the nuclei of target cells and stimulates the transcription of DNA and RNA, which is accompanied by increased synthesis of specific proteins.
If we take the sun from time to time, we do not need to look for it in our diet. It acts together with the hormonal parathoride and calcitonin in the absorption of calcium and phosphorus. Pharmacological basis of therapy. Some researchers believed that the disease was caused by a lack of fresh air And sunlight; others argued that the disease depended on a factor in the diet.
Mellanby and Huldschinsky showed that both ideas were correct; adding cod oil to the diet or exposure to sunlight prevented or cured the disease. These observations led to the elucidation of the structures of collecalciferol and ergocalciferol and, ultimately, to the discovery that these compounds require additional processing in the body to become active. The main provitamin found in animal tissues is 7-dehydrocholesterol, which is synthesized in the skin. Exposure of the skin to sunlight converts 7-dehydrocholesterol to cholecalciferol.
However, the role of vitamin D is not limited to protecting bones; it affects the body's susceptibility to skin diseases, heart disease and cancer. In geographic areas where food is poor in vitamin D, the incidence of atherosclerosis, arthritis, and diabetes, especially juvenile diabetes, is increased.
It prevents muscle weakness, improves immunity (the level of vitamin D in the blood is one of the criteria for assessing the life expectancy of AIDS patients), and is necessary for the functioning of thyroid gland and normal blood clotting.
The final activation of calcitriol occurs primarily in the kidneys, but also occurs in the placenta and kidneys. deciduas, as well as in macrophages. Soils are the predominant source of calcitriol in the circulation. The enzyme system responsible for 1-hydroxylation of 25-hydroxycholecalciferol is associated with mitochondria in the proximal tubule. Regulation is both chronic and acute. There is evidence that hypocalcemia can directly activate hydroxylase, as well as indirectly influence it by causing the secretion of parathyroid hormone.
Thus, with external use of vitamin D 3, the scaly skin characteristic of psoriasis decreases.
There is evidence that, by improving the absorption of calcium and magnesium, vitamin D helps the body restore the protective membranes surrounding the nerves, so it is included in the complex therapy of multiple sclerosis.
Vitamin D 3 is involved in the regulation of blood pressure (in particular, with hypertension in pregnant women) and heartbeat.
Hypophosphatemia significantly increases hydroxylase activity. Calcitriol exerts negative feedback control of the enzyme, reflecting a direct effect on the kidneys, as well as inhibition of parathyroid hormone production. The nature of the regulatory mechanisms of estrogen and prolactin on 1a-hydroxylase is unknown. The vitamin affects phosphate metabolism in parallel with the Ca 2 method. The mechanism of action of calcitriol is similar to the mechanism of action of steroid and thyroid hormones. Structural analysis of the calcitriol receptor indicates that it belongs to the same family of supergenes as the steroid and thyroid hormone receptors.
Vitamin D inhibits the growth of cancer cells, making it effective in the prevention and treatment of breast, ovarian, prostate, brain cancer, and leukemia.
Hypovitaminosis. Lack of vitamin D in children leads to rickets. The main manifestations of this disease are reduced to symptoms of calcium deficiency. First of all, osteogenesis suffers: there is deformation of the skeleton of the limbs (their curvature as a result of softening - osteomalacia), the skull (late fusion of the fontanelles), chest(appearance of a kind of “rosary” on the osteocartilaginous border of the ribs), teething is delayed. Muscle hypotonia develops (enlarged abdomen), neuromuscular excitability increases (in a baby, a symptom of baldness of the back of the head is detected due to frequent rotation of the head), seizures may occur. In an adult, calcium deficiency in the body leads to caries and osteomalacia; in the elderly - to the development of osteoporosis (decrease in bone density due to impaired osteosynthesis). The destruction of the inorganic matrix is explained by the increased “leaching” of calcium from bone tissue and impaired reabsorption of calcium in the renal tubules due to vitamin D deficiency.
Calcitriol also appears to have effects that occur so quickly that they are interpreted as events that are too rapid, which is explained by genomic actions. Figure 17: Structures of 7-dehydrocholesterol, ergosterol, cholecalciferol and ergocalciferol.
Its deficiency causes rickets, osteoporosis and osteomalacia, with decalcification of the bone, which becomes deformed even if the same mass is maintained. Lack of growth in children and skeletal deformities in adults can occur during pregnancy and lactation, with symptoms of low back pain, spasm and muscle weakness, spinal and pelvic deformities.
The diagram below shows inhibition (dashed arrow) of absorption, decreased calcium entry into the bone and decreased calcium excretion with a lack of vitamin D. At the same time, in response to hypocalcemia, parathyrin is secreted and increases (solid arrow) the flow of calcium from the bone into the bloodstream (secondary hyperparathyroidism).
Symptoms of hypovitaminosis
The main symptom of vitamin D deficiency is rickets and softening of the bones (osteomalacia).
Milder forms of vitamin D deficiency include symptoms such as:
loss of appetite, weight loss,
burning sensation in the mouth and throat,
insomnia,
blurred vision.
Rickets, one of the most common childhood diseases, has been known since time immemorial. Paintings by Flemish artists depicting children with twisted spines, arms and legs clearly indicate the spread of rickets in the 15th century. Rickets became widespread in Great Britain - it was also called the “English disease.” As it became known later, ultraviolet light is needed to activate the anti-rachitic vitamin, so foci of rickets became big cities with dense buildings and smoke. With rickets, the most pronounced disorders are in the bones of the legs, chest, spine and skull. Cartilage and bone tissue become abnormally soft, which leads to their deformation and curvature. The disease rickets is possible even with sufficient vitamin content in food, but if its absorption in the digestive tract is impaired (digestive disorders at an early age).
With a lack of vitamin D in animals, the content of calcium and phosphorus in the blood decreases, appetite disappears, the functioning of the respiratory system is disrupted, growth is delayed, softening of the limbs and brittle bones appear. Sometimes muscle cramps occur in the head, neck and limbs. Rickets is most pronounced in young animals. It has long been known that rickets can be treated well with fish oil.
Hypervitaminosis D. Hypervitaminosis D is quite dangerous (occurs at doses many times higher than therapeutic doses), because this causes hypercalcemia of the body and calcification of internal organs: kidneys, stomach, lungs, large blood vessels. Excess vitamin D is deposited in the liver and can cause poisoning.
Excessive intake of vitamin D leads to intoxication and is accompanied by severe demineralization of bones - up to their fractures. The calcium level in the blood increases. This leads to calcification of soft tissues, the kidneys are especially prone to this process (stones form and kidney failure develops). The increase in the level of calcium (and phosphorus) in the blood is explained by the following: 1) resorption of bone tissue (solid arrow); 2) an increase in the intensity of absorption of calcium and phosphorus in the intestine 3) an increase in their absorption in the kidneys (i.e., inhibition of excretion in the urine - dotted line).

IN normal conditions an increase in calcium levels in the blood will lead to the formation of inactive 24.25(0 H)2-D3, which does not undergo bone resorption (“resorption”), however, with hypervitaminosis D this mechanism becomes ineffective. Interestingly, skin pigmentation (tanning) is a protective factor that protects against excessive formation of vitamin D during UV irradiation of the skin. However, light-skinned residents of northern countries who lack sun exposure generally do not develop vitamin D deficiency because their diet includes fish oil.
Metabolism. Vitamins of group D are absorbed like vitamin A. In the liver, vitamins undergo hydroxylation by the microsomal oxygenase system at C-25 (25(OH)-D3 is formed from vitamin D, i.e. 25-hydroxycholecalciferol), and are then transported by the bloodstream using a specific transport protein to the kidneys. In the kidneys, the second hydroxylation reaction at C-1 occurs with the help of mitochondrial oxygenases (1,25(OH)2-D3 is formed, i.e. 1,25-dihydroxycholecalciferol, or calcitriol). This response is activated by parathyroid hormone, secreted by the parathyroid gland when calcium levels in the blood decrease. If the level of calcium is adequate to the physiological needs of the body, secondary hydroxylation occurs at C-24 (instead of C-1), resulting in the formation of an inactive metabolite 1,24(OH)2-D3. Vitamin C takes part in hydroxylation reactions.

Vitamin D3 accumulates in adipose tissue. It is excreted mainly in feces in unchanged or oxidized form, as well as in the form of conjugates.
Vitamin H - Biotin, coenzyme R
Vitamin H water soluble, relatively new member of the family B vitamins.
Biotin is needed for the synthesis of ascorbic acid. Necessary for normal metabolism of fats and protein.
RNI for adults 150 – 300 mcg. Can be synthesized by intestinal bacteria. Raw eggs interfere with its absorption by the body. Synergistic with vitamins B2, B6, niacin, A and keeps skin healthy.
Benefit: Helps protect hair from graying. Relieves muscle pain. Reduces the manifestations of eczema and dermatitis.
Diseases caused by biotin deficiency: impaired fat metabolism.
Best Natural Sources: nuts, fruits, brewer's yeast, beef liver, milk, kidneys and brown rice, egg yolk.
In 1935-1936 Kogi and Tonnies were the first to isolate crystalline biotin from egg yolk. For this purpose, they used 250 kg of egg yolks and obtained 100 mg of biotin with a melting point of 148°.
Amino acid derivatives of biotin are known, among which the most
Biocytin, which has high activity for many microorganisms, has been studied.
It is a peptide of biotin and lysine. The cause of pathological changes that occur when animals are fed raw has now been elucidated. egg white. It contains avidin protein, which specifically combines with biotin (introduced orally with food or synthesized by intestinal microorganisms) into an inactive complex and thereby prevents its absorption.
A record amount (6.81 µg/g) was found in the liver of a shark.
The liver, kidneys, and adrenal glands are richest in vitamins; the heart and stomach contain average, and brain tissue, lungs and skeletal muscles contain minimal amounts of biotin.
The richest in vitamins are pork and beef liver, kidneys, ox heart, egg yolk, and among plant products - beans, rice bran, wheat flour and cauliflower. In animal tissues and yeast, biotin is found predominantly in a protein-bound form; in vegetables and fruits, it is found in a free state.
Biotin biosynthesis.
Biotin biosynthesis is carried out by all green plants and some bacteria.
and mushrooms. The study of the biosynthesis pathways of biotin began after the structure of its molecule was elucidated. The chemical breakdown of biotin proceeds through the formation of desthiobiotin, diaminopelargonic acid and, finally, pimelic acid.
Interaction with other vitamins.
A connection has been established between biotin and other vitamins, in particular with folic acid, vitamin B12 - ascorbic acid, thiamine and pantothenic acid. A particularly close relationship exists between biotin and folic acid. Biotin has a beneficial effect on the general condition of the body and the preservation of ascorbic acid in the tissues of scurvy guinea pigs. In turn, ascorbic acid slows down, although does not prevent, the development of biotin deficiency in rats.
With biotin deficiency, the thiamine content in the liver and spleen decreases.
kidneys and brains of animals. Rats fed a diet lacking biotin had higher levels of vitamin B12 than control animals fed biotin. These two vitamins are closely related to each other in the metabolism of propionic acid in microorganisms and animals. There is a close connection between the biosynthesis of biotin and pantothenic acid in microorganisms and green plants (V.V. Filippov, 1962). Biotin alleviates the symptoms of pantothenic deficiency and, conversely, pantothenic acid mitigates the manifestation of biotin vitamin deficiency. Biotin avitaminosis
In rats, biotin deficiency develops after 4-5 weeks of feeding the experimental diet, and in chickens, the first signs of vitamin deficiency appear after 3 weeks.
Besides external signs, biotin vitamin deficiency causes profound morphological changes in tissues and organs, as well as metabolic disorders. Changes in the thymus gland, skin and muscles of rats are known. Characterized by abundant hyperkeratosis, acanthosis and edema. Destroyed hair shafts are mixed with hyperkeratotic plates. The expansion of the hair follicles was established, the openings of which were blocked by hyperkeratotic material. In the last phase of development of vitamin deficiency, fat atrophy is observed in hyperkeratotic plates. A lack of biotin in the diet of rats leads to a decrease in its content in tissues. In the liver and muscles, the amount of vitamin decreases by 5 times, and in brain tissue by 15%. Pyruvic acid accumulates in the blood of vitamin deficient rats, acidosis develops and sugar concentration decreases. In this case, glycosuria is not observed, but the content of reducing sugars in the liver decreases while their content in the muscles is normal; animals develop creatinuria.
A person completely satisfies his need for biotin due to its synthesis by intestinal microflora, so hypovitaminosis can only be obtained in an experiment.
Hypovitaminosis can develop mainly due to intestinal dysbiosis, which occurs, for example, as a result of taking antibiotics.
Symptoms of hypovitaminosis
Possible consequences of biotin deficiency: seborrheic dermatitis, anemia, depression, hair loss, high blood sugar, inflammation or paleness of the skin and mucous membranes, insomnia, loss of appetite, muscle pain, nausea, inflammation of the tongue, dry skin, high blood cholesterol .
Interaction
* Raw egg whites contain a substance called avidin, an anti-vitamin of biotin. This substance binds biotin and prevents its absorption into the blood. Heating denatures (irreversibly destroys the structure) the avidin in egg whites, so cooked eggs do not interfere with the absorption of biotin.
* Alcohol impairs the ability to absorb biotin, and therefore chronic alcohol abuse can lead to biotin deficiency.
* Oil fats that have been cooked or exposed to air for long periods of time will slow down the absorption of biotin.
* Antibiotics, medications containing sulfur and saccharin also affect the absorption of biotin.
If you need long-term antibiotic treatment - this applies to both children and adults - biotin synthesis may be sharply reduced due to the death of beneficial gut bacteria, making additional supplementation necessary.
Vitamin H is indicated for hair loss and psoriasis; in cosmetics it is used in hair care products and in masks.
Metabolism Protein-bound biotin comes from food, passes into a free state with the help of proteinases and is absorbed in the small intestine. When it enters the blood, it again combines with proteins (albumin) and enters the tissues. Biotin is retained mainly in the liver and kidneys. It is excreted unchanged in urine and feces. The coenzyme form of vitamin H is N5-carboxybiotin.
Structure and properties.
The structure of biotin is based on a thiophene ring, to which urea is attached, and the side chain is represented by valeric acid:
Biotin is a crystalline substance, highly soluble in water and alcohol. This is a stable compound, the biological activity of which does not change after boiling solutions and with access to oxygen.
Empirical formula: C 10 H 16 O 3 N 2 S.
prof. Kruglov Sergey Vladimirovich (left), Kutenko Vladimir Sergeevich (right)
Page editor: Kutenko Vladimir Sergeevich

Kudinov Vladimir Ivanovich
Kudinov Vladimir Ivanovich, Candidate of Medical Sciences, Associate Professor of Rostov State Medical University, Chairman of the Association of Endocrinologists of the Rostov Region, Endocrinologist of the highest category

Dzherieva Irina Sarkisovna
Dzherieva Irina Sarkisovna Doctor of Medical Sciences, Associate Professor, Endocrinologist
CHAPTER 4 VITAMIN METABOLISMDAND KIDNEYS
J. LEHMAN, R. W. GRAY
(J. LEMMANN, R. W. GRAY)
It has long been known that severe kidney disease is often accompanied by bone lesions, including rickets or osteomalacia, as well as slower absorption of calcium in the intestine. Rickets or osteomalacia in combination with slow absorption of calcium in the intestine are also signs of vitamin D deficiency. Over the past 15 years, as a result of the emergence of a lot of new information about the endocrine nature of vitamin D, the role of the kidneys in its metabolism has become clear. In addition, the need for treatment is all more patients with chronic renal failure before and during life-sustaining dialysis forced them to clinically evaluate this new information and widely use it in practical medicine. Below we summarize modern ideas about the metabolism and action of vitamin D, with special attention paid to the role of the kidneys in these processes and to the analysis of disorders of vitamin D metabolism in pathological conditions.
HISTORICAL SKETCH
The history of vitamin D began with the elucidation of possible population characteristics in the structure of the skeleton and the study of the pathogenesis of rickets as a bone disease. According to the description of Herodotus (484-425 BC), the killed Egyptian soldiers, who, according to custom, had not covered their heads from the sun since childhood, had hard skulls, while the Persian soldiers, who always wore turbans, had soft skulls. Such observations were taken as indications that sunlight could provide thickness and hardness to bones. Medical problem rickets only became evident in the 17th century, when in England and Northern Europe cities are formed. Soon, random observations appeared about the healing effect of fish oil on rickets. Unfortunately, the widespread use of this folk remedy was delayed for more than a century, until the understanding of the importance of substances present in trace amounts in food (in particular, vitamins) was formed.
By the end of the 19th century. Geographical studies again pointed to the prevalence of rickets in cities where the population lacked sunlight. A study of the skeleton of deceased patients with rickets made it possible to establish reduced mineralization of bones and a lower content of calcium and phosphorus in them, while metabolic studies revealed a low rate of absorption of calcium and phosphorus in the intestines of such patients.
By the end of World War I, two main steps had been taken towards understanding the nature of rickets. Mellanby developed a method of reproducing rickets in puppies, showing that the disease was experimentally cured by cod liver oil. Such observations clearly indicated the presence of an anti-rickets factor in the diet. Around the same time, Huldchinsky managed to cure children by irradiating them under a mercury lamp. Moreover, in 1 child, irradiation of only one arm resulted in the appearance of radiographic signs of improvement in the condition of the bones of the entire skeleton. This observation was the first clear evidence of the possibility of formation in the body of a circulating and apparently hormonal anti-rickets factor. Subsequently, experiments on rats with experimental rickets showed that irradiation of not only the rats themselves, but also their food, leads to a cure. The anti-rickets factor present in the diet turned out to be a fat-soluble substance, different from vitamin A. In 1932, the structure of vitamin D-secosterol, formed during irradiation of food products, was determined, and in 1936 the structure of natural vitamin D was determined. Taken together, these observations formed the basis of the modern concept that vitamin D is both a hormone and (in conditions of limited sunlight) a vitamin.

Structure of Vitamin D
CHEMICAL STRUCTURE OF VITAMIND
In Fig. Figure 39 shows the structure of vitamin D 3 - cholecalciferol, as well as the numbering system for the positions of carbon atoms in its molecule. Vitamin D 2 - ergocaldiferol - differs only in the presence of a double bond between the 22nd and 23rd carbon atoms and an additional methyl group at position 24. The molecular weight of vitamin D 3 is 384. It is insoluble in water, but easily soluble in organic solvents and fats . The vitamin is very sensitive to oxidation in air or by peroxides in solution and is destroyed in an acidic environment. A review has recently been published on the structure and configuration of vitamin D molecules and similar secosteroids.
VITAMIN METABOLISMD
A detailed elucidation of the metabolic pathways of vitamin D 3 became possible only after its chemical synthesis with an isotopic label, the development of chromatographic methods for separating its metabolites, and the creation of methods for biological assessment of the effects of both vitamin D 3 itself and its metabolites. 7-Dehydrocholesterol is formed in the skin under the action of an enzyme and under ultraviolet irradiation (mainly at a wavelength of 300-310 nm) is converted into previtamin D 3. The latter spontaneously undergoes temperature isomerization into vitamin D 3, which then combines with serum vitamin D-binding globulin (DVG). The complex of vitamin D 3 with protein is transported by the blood to the liver, where vitamin D 3 is hydroxylated at the 25th carbon atom, forming the main form of the vitamin in plasma - 25-OH-D 3. This substance is in turn transported by DSG to the kidneys, where it can undergo further hydroxylation into the hormonal form of vitamin D 3 -l,25-(OH) 2 -D 3.
Synthesis of vitamin D 3 in the skin
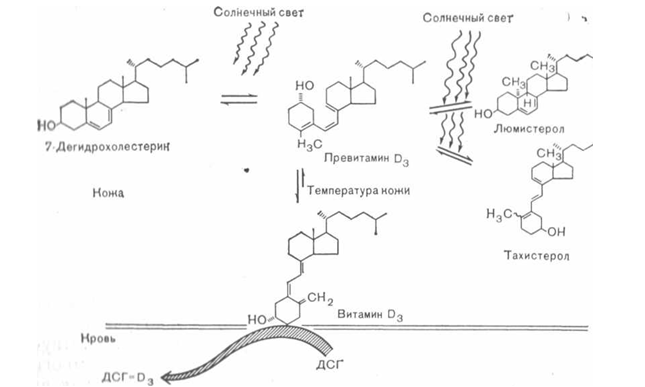
Vitamin D synthesis
Modern ideas about the synthesis of vitamin D 3 in the skin are schematized in Fig. 41. As already noted, previtamin D 3 is formed in the skin from 7-dehydrocholesterol. The skin contains large quantities of this precursor, due to which the rate of formation of the previtamin is not limited. Holick et al. irradiated human skin in vitro and found that exposure to light, simulating solar irradiation near the equator, can ensure maximum formation of previtamin D 3 in the basal cell layers of the skin within just a few hours. Some time ago, it was already suggested that dark pigmentation of the skin may completely exclude the synthesis of vitamin D in it. More recent studies by Holick et al. showed that with an increase in skin pigmentation, the period of light exposure required for maximum formation of previtamin D3 increases. This inhibition of previtamin D3 production could therefore reduce vitamin D3 synthesis in highly pigmented skin under conditions of limited sunlight. Previtamin D 3 then undergoes a temperature-dependent non-enzymatic conversion in the skin and vitamin D 3 . It takes approximately 1 day to convert 50% of the provitamin Da present in the skin into vitamin D3. After this, vitamin D 3 binds to DSG and enters the blood. The details of the mechanism of formation of the DSG-D 3 complex and its penetration into the blood remain unknown.
Transport of vitamin D and its metabolites: vitamin D-binding globulin
Vitamin D-binding globulin is synthesized in the liver and is identical to the human group-specific protein (Gc), long known to geneticists. Genetic variants of this protein do not appear to differ in their ability to bind vitamin D sterols. Since the total concentration of all vitamin D metabolites in serum does not normally reach 10 -7 M (see below), and the molar concentration of DSG is approximately two orders of magnitude above, it is obvious that such an excess of binding protein contributes to the preservation of vitamin D and its metabolites under conditions of limited intake into the body and prevents the toxicity of vitamin D when its consumption or synthesis in the skin increases. DSG not only participates in the transport of vitamin D metabolites in the blood, but is also present in the form of a complex with cytosolic protein in the cells of many tissues. Recently this protein was identified as actin. It is therefore assumed that DSG may be involved in the transfer of secosteroids from the extracellular fluid into the cells. On the other hand, the main determinant of steroid transport into cells may be the concentration of free (non-DSG-related) vitamin D metabolites.
Synthesis of 25-OH-D 3 in the liver
Vitamin D circulating in the blood is quickly taken up by the liver, where it undergoes hydroxylation at the 25th carbon atom to form 25-OH-D 3 . Hydroxylation is carried out mainly by a mixed-function microsomal monooxygenase and requires the presence of molecular oxygen, flavoprotein and cytochrome P-450. The km of this enzyme is approximately 10 -8 M. The activity of microsomal hydroxylase decreases with the introduction of vitamin D 3 . In addition, the liver also contains mitochondrial 25-hydroxylase, which requires iron-sulfur-containing protein and cytochrome P-450 as cofactors, but has a much higher Km (approximately 10 -6 M). This indicates the importance of this enzyme in the production of 25-OH-D 3 only in the presence of unusually high concentrations of vitamin D 3 . 25-Hydroxylation is also found in the intestines and kidneys of birds, but in terms of quantity these organs probably play only a minor role in the total production of 25-OH-D 3 .
Further metabolism of 25-OH-D 3 can also occur in the liver with the formation of more polar and biologically inactive products, which is observed mainly under conditions of accelerated microsomal hydroxylation caused by various pharmacological agents.
Synthesis 1.25-(OH) 2 — D 3 and 24,25-(OH) 2 — D 3 in the kidneys
Further metabolism of 25-OH-D 3 occurs mainly in the kidneys. The main metabolites are l,25-(OH) 2 -D 3 and 24,25-(OH) 2 -D 3 . l,25-(OH) 2 -D 3 is synthesized by 25-OH-D 3 -1 α -hydroxylase, a mixed-function mitochondrial monooxygenase, which in mammals is apparently present only in the proximal tubules, but in birds it is not contained only in the proximal tubules, but also in the glomeruli. This enzyme is composed of several components, including iron-sulfur protein associated with NAD, flavoprotein, and cytochrome P-450 specific for 25-OH-D 3 . A specific component of the system (cytochrome P-450) introduces one oxygen atom into position 1 α. This entire enzyme system has been studied almost exclusively in chicken kidneys or in primary cultures of chicken kidney cells. Its K m ranges from 1.2 to 3.6x10 -7 M, and V max is approximately 5.5 mol/mg of mitochondrial protein per minute. In the mammalian kidney, this enzyme is more difficult to study, probably due to the presence of large quantities of 25-OH-D 3 -binding proteins, which limits the availability of the substrate. Recent studies, however, have found 1 α-hydroxylase activity in mitochondria isolated from the renal cortex of rats with D-vitaminosis. The K m of the enzyme (8.9x10 -7 M) is slightly higher than for the mitochondrial enzyme in chickens, although the latest data need to be confirmed. The enzyme was also found in kidney sections of rats, in isolated rat kidney cells, and in cultured mouse kidney cells. In most of these studies, enzymatic activity was determined by the conversion of labeled 25-OH-D 3 to radioactive products that migrated with the authentic l,25-(OH) 2 -D 3 on high-performance liquid chromatography. Chemically, this product was identified as 1,25-(OH) 2 -D 3 only in rat kidney homogenes.
In the kidney, 24,25-(OH) 2 -D 3 is also formed from 25-OH-D 3 . This occurs under the action of an enzyme that is also a mixed-function mitochondrial oxidase and is localized in rats, apparently, in the proximal convoluted and straight renal tubules. The 24-hydroxylase km of mitochondrial preparations of chicken kidney is approximately 1x10 -6 M, and that of rat kidney is about 3.8x10 -7 M.
Extrarenal synthesisl,25-(OH) 2 — D 3 and 24.25-(OH) 2 — D 3
After it was shown that in the body of pregnant and nephrectomized rats with vitamin deficiency D, labeled 25-OH-D 3 can be converted into a more polar metabolite that migrates along with l,25-(OH) 2 -D 3, it was possible to clarify the role of the placenta in metabolism of 25-OH-D 3 to form a product chemically identified as l,25-(OH)2-D 3 . Recently, data have also been published showing that fetal rat skull roof cells in culture are capable of synthesizing a substance that migrates with l,25-(OH) 2 -D 3 . In contrast to all these observations, the absence of detectable amounts of l,25-(OH) 2 -D 3 in the blood serum of kidneyless people maintained on dialysis, as well as in nephrectomized non-pregnant animals, has been repeatedly reported. More recently, however, it was possible to determine a low concentration of l,25-(OH) 2 -D 3 in the blood serum of kidney-deprived patients. Moreover, the administration of vitamin D2 to such patients led to an increase in the level of l,25-(OH) 2 -D 3 in the serum (but not to normal). Although these observations indicate that in humans, in conditions of availability of large quantities of the precursor (25-OH-D), some l,25-(OH) 2 -D can be formed outside the kidneys, however, the normal level of l,25-(OH ) Serum 2-D is independent of 25-OH-D concentration. Nevertheless modern research give reason to believe that in certain conditions (in children and people with vitamin D deficiency, exposed ultraviolet irradiation) the level of l,25-(OH)2-D may still depend on the concentration of the precursor (25-OH-D).
The kidney appears to be the primary organ where 24,25-(OH)2-D is synthesized in humans, as serum levels decrease as the disease progresses. renal failure, becoming very low and often undetectable in patients without kidneys. However, data have been obtained in animals showing that other tissues, including the intestine and cartilage, can form a labeled metabolite from 3 H-25-OH-D 3, which has the same chromatographic properties and sensitivity to the destructive effect of periodate as genuine 24,25-(OH) 2 -D 3 . Additionally, 24,25-(OH)2-D3 has been chemically identified in the plasma of nephrectomized pigs given high doses of vitamin D. Similarly, 24,25-(OH)2-D levels in the serum of kidney-deprived humans increase when they receive large doses of vitamin D 2 . Thus, with an excessively high concentration of 25-OH-D in human serum, 24,25-(OH) 2 -D can be synthesized outside the kidneys.
Enterohepatic circulation of vitamin metabolitesD
Vitamin D and its metabolites are excreted mainly in feces. Vitamin D 3 , 25-OH-D 3 and l,25-(OH) 2 -D 3 are conjugated in the liver and secreted into bile. There is also evidence that these metabolites can be reabsorbed and reutilized, thus forming a “reserve” mechanism for vitamin D metabolism.
Other vitamin metabolitesD
25-OH-D 3 can be converted not only into l,25-(OH) 2 -D 3 and 24,25-(OH) 2 -D 3, but also into other compounds. These include 25,26-(OH) 2 -D 3 and 25-OH-D 3 -26,23-lactone. These metabolites, like 24,25-(OH) 2 -D 3 , can undergo 1-hydroxylation, forming 1,24,25-(OH) 3 -D 3 , 1,25,26-(OH) 3 -D 3 and l,25-(OH) 2 -D 3 -26,23-lactone. In addition, the side chain 1,25-(OH)2-D 3 may undergo oxidation, resulting in the formation of a 23-acid (calcitroic acid); when the side chain 25-OH-D 3 is oxidized, a 24-acid (cholacalcic acid) is formed. Later, 23,25-(OH) 2 -D 3 , 25-OH-24-oxo-D 3 , 25-OH-trans-D 3 and 19-nor-10-oxo-25-OH-D 3 were isolated. These metabolites, in turn, can undergo further transformation. Current evidence suggests that the biological effects of these metabolites do not correspond to those of 1,25-(OH) 2 -D 3 , so their physiological role remains unclear. Some of them (if not all) are probably degradation products.
BIOLOGICAL EFFECTS OF VITAMIND
Transport processes in the intestine
Calcium
Calcium absorption in the intestine occurs through active transport against an electrochemical gradient, and also (when the calcium content of food and, consequently, its concentration in the intestinal lumen increases excessively) through passive movement. In animals and humans with vitamin D deficiency, the absorption of calcium in the intestine under conditions of normal dietary intake is reduced. Administration of vitamin D3 to animals with vitamin D deficiency restores the normal rate of calcium absorption in the intestines no earlier than after 16 hours. The existence of this lag period allowed us to assume and then prove that vitamin D must undergo some transformations, and in the intestines must occur some changes before calcium transport normalizes. It has long been shown that 25-OH-D 3 normalizes calcium transport faster than vitamin D 3 does. l,25-(OH) 2 -D 3 was then identified and found to be a more effective and faster-acting metabolite of vitamin D in stimulating intestinal calcium absorption. During the period of maximum stimulation of active transport caused by the administration of labeled vitamin D 3 to animals with vitamin D deficiency, only labeled 1,25-(OH) 2 -D 3 can be detected in the intestine. This compound apparently binds to a specific receptor protein in the cytosol of intestinal cells, which transfers the steroid to the nucleus, where it complexes with chromatin and induces protein synthesis, similar to what happens with other steroid hormones. In response to the action of 1,25-(OH) 2 -O 3, a specific mRNA is formed, encoding the synthesis of a specific calcium-binding protein (CaSB). The content of the latter in the intestine correlates with calcium transport in this organ, increasing when animals with vitamin D deficiency are administered vitamin D 3 or as active calcium transport increases when calcium is restricted in the diet, and decreasing as calcium absorption in the intestine slows down, which occurs with age. These data suggest that CaSB takes part in the active transport of calcium in the intestine as a carrier. On the other hand, CaSB appears in the intestine somewhat later than the stimulation of calcium transport occurs caused by the administration of 1,25-(OH) 2 -D 3 to animals with vitamin deficiency D. In addition, an increase in calcium absorption in the intestine under such conditions occurs due to Apparently, it is two-phase: after the initial transient stimulation of the process, its repeated and prolonged acceleration is recorded. Thus, CaSB can not so much “trigger” calcium transport as maintain it at an elevated level. Preliminary administration of protein synthesis inhibitors to animals with vitamin D deficiency prevents the effect of 1,25-(OH) 2 -O 3 on the synthesis of CaSB in the intestine, but does not block the restoration of calcium absorption. Later, it was suggested that l,25-(OH) 2 -D “triggers” calcium transport by changing the phospholipid composition of the cell membrane facing the intestinal lumen. Vitamin D can also accelerate the passive movement of Ca in the intestine, since the intake of Ca when vitamin D is administered to animals with D-beri deficiency also increases in that case; when the intestine is examined at low temperatures or under anaerobic conditions.
In humans, under conditions of vitamin D saturation, intestinal calcium absorption, determined either as true (using isotope techniques) or as resulting (using balance experiments), directly correlates with l,25-(OH) 2 -D concentrations in the range from zero to the upper limit of normal. This dependence is unusually sensitive: in individuals on a normal diet providing 10-25 mmol of calcium per day, with an increase in the concentration of l,25-(OH) 2 -D in the blood serum by 1 pM, calcium absorption in the intestine increases by 0. 23%, or 0.06 mmol. Thus, with a normal calcium content in the diet, its absorption in the intestine is determined by the availability of l,25-(OH) 2 -D.
Phosphate
With vitamin D deficiency, phosphate transport in the intestine is also reduced. The introduction of vitamin D or 1,25-(OH) 2 -D 3 under such conditions enhances the intestinal absorption of phosphate. This effect is apparently due to the presence of l,25(OH) 2 -D 3, since 25-OH-D 3 does not stimulate phosphate transport when administered to nephrectomized animals with vitamin deficiency D. The greatest stimulation of phosphate absorption after administration of l, 25-(OH) 2 -D is observed in the jejunum; then - in the duodenum and ileum. But although there is still controversy on this issue, it appears that calcium is required for vitamin D to stimulate phosphate absorption. Administration of l,25-(OH) 2 -D 3 can enhance intestinal absorption of phosphate in humans. However, in patients without kidneys and on hemodialysis, in whom the concentration of l,25-(OH) 2 -D in the blood serum cannot be determined, a significant proportion of the phosphate contained in food is absorbed. This indicates that intestinal absorption of phosphate is independent of l,25-(OH) 2 -D 3 . In people under conditions of vitamin D saturation, the absorption of phosphate in the intestine, expressed as a percentage of its intake from food (with normal fluctuations of the latter), increases by 0.05% per 1 pM increase in the concentration of l,25-(OH) 2 -D.
Although the most effective and fast-acting metabolite of vitamin D in terms of intestinal absorption of calcium and phosphate is 1,25-(OH) 2 -D 3, other metabolites of this vitamin may also have a certain activity in pharmacological doses. Currently, however, it is believed that l,25-(OH) 2 -D is the only physiologically significant form of vitamin D for the intestinal absorption of these minerals.
Bone
Bone damage (rickets during growth and osteomalacia in adults) is a pathognomonic sign of D-vitaminosis. Bone pathology includes failure of calcification and resorption of epiphyseal cartilage, as well as loss of epiphyseal bone formation along with metaphyseal proliferation of unmineralized osteoid or bone matrix. When treating animals and people with D-beri deficiency with the appropriate vitamin, normalization of epiphyseal cartilage calcification is observed and normal bone growth occurs in combination with osteoid mineralization, which leads to the disappearance of bone pathology. As for the mechanisms of the listed effects of vitamin D on bone, there are still conflicting opinions. On the one hand, it is assumed that normal bone mineralization is caused by increased concentrations of calcium and phosphate in the serum and extracellular fluid, which in turn is a consequence of the stimulating effect of vitamin D on the absorption of calcium and phosphate in the intestine. On the other hand, vitamin D or one of its metabolites could act directly on cartilage and bone tissue, normalizing their mineralization. Although vitamin D overdose is known to cause skeletal demineralization and l,25-(OH) 2 -D in vitro increases bone resorption, there is evidence that vitamin D can stimulate bone mineralization. For example, in patients with osteomalacia caused by chronic renal failure, artificially increasing the levels of calcium and phosphate in the blood serum does not normalize bone mineralization, whereas such normalization occurs with the introduction of vitamin D. The mechanism by which vitamin D directly affects bone remains unknown. However, bone cells contain specific l,25-(OH) 2 -D receptors, which theoretically could mediate this effect.
In addition, there is controversy regarding the nature of the vitamin D metabolite required for normal bone mineralization. In some clinical studies it was shown that when l,25(OH) 2 -D 3 is administered alone to patients with vitamin D deficiency, only mineralization defects disappear, but excess amounts of osteoid remain. To completely eliminate bone pathology in these studies, vitamin D 3, or 25-OH-D 3, or a combination of l,25-(OH) 2 -D 3 with 24,25-(OH) 2 -D 3 was administered. It follows that 24,25-(OH) 2 -D might be required for normal bone formation. Later, however, reports appeared that the introduction of l,25-(OH) 2 -D 3 alone could lead to complete cure osteomalacia, despite maintaining low levels of 25-OH-D and 24,25-(OH) 2 -D in the blood serum. Moreover, recent studies on experimental rickets in animals have shown that the elimination of bone pathology can be achieved by introducing an analogue of vitamin D-24,24-difluoro-25-OH-D 3, which does not undergo hydroxylation at the 24th position, but is capable of hydroxylation at 1st position. The data presented argue against the role of 24,25-(OH) 2 -D 3 deficiency in the pathogenesis of rickets and osteomalacia. For final decision This issue obviously requires further research.
Kidneys
Specific cytosolic l,25-(OH) 2 -D 3 receptors with high affinity for this compound have been identified in the kidneys. However, the effects of vitamin D and its metabolites on the kidney remain an area of controversy. In studies of renal clearance, it has long been shown that 25-OH-D 3 and 1,25-(OH) 2 -D 3 enhance tubular reabsorption of calcium and phosphate in dogs, which should help preserve the reserves of these substances in the body. However, subsequent clearance studies in thyroidectomized rats receiving physiological doses of l,25-(OH) 2 -D (which restored intestinal calcium absorption to normal) did not reveal changes in calcium excretion per unit glomerular filtration rate as concentrations progressively increased serum calcium to normal or higher. In contrast, parathyroid hormone (PTH) decreased urinary calcium excretion as expected. Such observations would indicate that l,25-(OH) 2 -D has no apparent effect on renal tubular calcium transport. However, further study of this issue clearly demonstrated the importance of l,25-(OH) 2 -D for calcium transport in other tissues where l,25-(OH) 2 -D and CaSB are present, as well as the presence of l,25-(OH) receptors ) 2-D and CaSB in the kidneys. In similar experiments determining the dynamics of phosphate in the kidneys of thyroid-parathyroidectomized rats treated with 1,25-(OH) 2 -D 3, inhibition of tubular reabsorption of phosphate was shown. This effect appears to be mediated by increased phosphate secretion from the more proximal segments of the tubule and possibly from the segment outside the distal convoluted part. Moreover, the administration of l,25-(OH) 2 -D to patients with hypoparathyroidism is accompanied by a decrease in the level of phosphate in the blood serum. Taken together, these data thus indicate the ability of vitamin D to increase urinary phosphate excretion, an effect unrelated to the well-known phosphaturic effect of PTH.
In humans, an increase in the concentration of l,25-(OH) 2 -D in serum is accompanied by an increase in calcium excretion in the urine. Under conditions of normal calcium intake, this effect is apparently a consequence of increased intestinal absorption and increased serum concentrations, which leads to an increase in glomerular filtration of calcium and (as a result of a decrease in the level of immunoreactive PTH in the serum) to inhibition of tubular reabsorption. With a low calcium content in the diet, an increase in the level of 1,25-(OH) 2 -D in the blood serum is also accompanied by increased excretion of calcium in the urine due to its more efficient absorption in the intestine and bone tissue resorption, but the calciuric reaction is still less pronounced than in conditions of normal calcium intake.
Other fabrics
Having high affinity for l,25-(OH) 2 -D receptors and/or vitamin D-dependent CaSB are found not only in the intestines, bones and kidneys, but also in the mammary glands, skin, parathyroid glands, pituitary gland and pancreas. In addition, receptors have been found in cultured human fibroblasts and some malignant cell lines.
The l,25-(OH) 2 -D 3 receptors have a sedimentation constant of approximately 3.3S and have a high affinity for the hormone: Kd is about 10 -10 M. The presence of 1,25-(OH) 2 -D receptors and CaSB in the mammary glands is apparently consistent with the presence of Ca transport into milk. As for other tissues, they are not generally recognized targets of vitamin D and its metabolites, so the role of the latter in regulating their function remains unknown. In the skin, 1,25-(OH)2-D could be expected to regulate vitamin D production. 1,25-(OH)2-D could also facilitate calcium entry into the parathyroid glands, thereby enhancing its known inhibitory effect on PTH secretion. Similarly, by facilitating calcium entry into pancreatic β-cells, l,25-(OH)2-D could enhance insulin secretion.
Possible role 24,25-(OH) 2 — D
After the discovery of the chemical synthesis of 24,25-(OH) 2 -D 3, interest arose in its possible biological role, since it is the main metabolite of vitamin D not only in animals, but also in humans. The first experiments on animals with D-vitaminosis showed that the biological activity of 24,25-(OH) 2 -D 3 is comparable to that of its predecessor - 25-OH-D 3. However, this activity appears to be primarily due to lα-hydroxylation of the metabolite and the formation of 1,24,25-(OH) 2 -D 3 . Studies conducted in healthy individuals and kidney-deprived patients showed that the administration of 24,25-(OH) 2 -D could stimulate calcium absorption in the intestine, but this could not be confirmed in subsequent observations. It was later suggested that 24,25-(OH) 2 -D 3 is necessary for the normal hatching of chicken eggs and is involved in the process of bone formation. This hypothesis is supported by the data that 24,25-(OH) 2 -D increases the synthesis of proteoglycans in cultured chondrocytes from growth zones and that the nuclei of these cells contain specific binding sites for 24,25-(OH) 2 -D. However, as noted above, the administration of l,25-(OH) 2 -D to people with D-vitaminosis can lead to a complete cure of osteomalacia, as is observed in bone pathology in rats with vitamin D deficiency.
24,25-(OH)2-D has also been reported to inhibit PTH secretion in dogs, and also to reduce the weight of hypertrophied parathyroid glands in vitamin D-deficient chickens. In addition, the presence of specific 24,25-(OH) receptors has been reported. 2-D in the parathyroid glands of chickens with rickets. However, Scatchard analysis has not been performed, so these receptors have not yet been characterized with sufficient certainty. Thus, although the important role of 24,25-(OH) 2 -D in calcium homeostasis is quite possible, this hypothesis remains speculative and requires additional confirmation.
REGULATION OF VITAMIN METABOLISMD
Plasma concentrations of vitamin D and its metabolites reflect the total amount of vitamin D 3 and D 2 metabolites, since the binding experiments on which these data are based generally did not distinguish between these forms. The main form of vitamin D present in plasma is 25-OH-D; its average concentration is about 60 nM, or 25 ng/ml. The concentrations of vitamin D itself and 24,25-(OH) 2 -D are approximately 10 times lower. The concentration of l,25-(OH) 2 -D is approximately 1000 times lower than 25-OH-D. The half-life of vitamin D in plasma is about 1 day, as it is rapidly converted to 25-OH-D (which is the same as vitamin D 3). The plasma half-life of 25-OH-D, judging by the results of determining the rate of disappearance of administered 3 H-25-OH-D 3 and the slope of the curve, the decline in the level of 25-OH-D 3 after the administration of its pharmacological; doses is approximately 3 weeks. Therefore, plasma 25-OH-D levels appear to allow a more accurate assessment of vitamin D reserves in the body. The plasma half-life of 1,25-(OH) 2 -D 3 is very short. 3 H-l,25-(OH) 2 -D 3 introduced into the blood disappears from the plasma with a half-life of less than 10 minutes, although the half-life of l,25-(OH) 2 -D disappears from the plasma after its administration in both healthy adults and in kidney-deprived patients it is approximately 6 hours (R. W. Gray, J. Lemann, unpublished data). Therefore, the level of l,25-(OH) 2 -D in plasma primarily reflects the production of this steroid hormone.
25-OH-D 3
The synthesis of 25-OH-D 3 in the liver does not appear to be under strict control. The concentration of a given substance in serum, which serves as best indicator Vitamin D reserves in the body depend mainly on the consumption of this vitamin and its synthesis in the skin under the influence of sunlight. In England, where vitamin D2 is not added to milk, serum 25-OH-D concentrations in healthy individuals range from about 30 nM in late winter to 60 nM in late summer, which is related to the intensity of solar exposure. Similar, although less pronounced, seasonal variations in serum 25-OH-D levels were noted in the United States, where its minimum concentration in winter is higher, probably due to the widespread addition of vitamin D 2 to milk and, more importantly, more high level 25-OH-D 3 in summer. The latter may be partly explained by the retention of previtamin D3 in the skin, since DSG has a much greater affinity for vitamin D3 than for previtamin D3. Artificial irradiation of 600 cm 2 of skin for approximately 15 minutes over 2 weeks. increases the content of 25-OH-D in serum by 4-11 nM. Calculations show that each square centimeter of irradiated skin could produce up to 0.024 nmol of 25-OH-D 3 . It follows that solar exposure to even limited areas of the skin could be the most important determinant of serum 25-OH-D levels and total body vitamin D stores. The latter, therefore, depend mainly on geographical latitude residence, season of the year and lifestyle.
Metabolism and excretion of 25-OH-D 3 were studied in healthy adults following injections of 3 H-25-OH-D 3 . In 1 week approximately 10% of the substance is excreted in the urine (almost exclusively in the form of water-soluble conjugates of its metabolites); another 15% is excreted in feces, also mainly in the form of conjugates and metabolites. It is believed that 25-OH-D 3 is also metabolized by side chain oxidation.
l,25-(OH)2-D3
Since the renal 25-OH-B3-1a-hydroxylase complex is localized in mitochondria, the regulation of 1,25-(OH)2-Oz synthesis by this enzyme system suggests the existence of a sequence of signals. Hormonal or neural regulatory stimuli would act on cell membrane, generating "second messengers" such as cyclic AMP, or changing the intracellular concentration of ions or substrates and cofactors required for energy production in mitochondria.
Vitamin D deficiency in humans is naturally accompanied by a sharp decrease in serum concentrations of 25-OH-D, 24,25-(OH) 2 -D and l,25-(OH) 2 -D. These patients also have hypocalcemia. to varying degrees severity, secondary hyperparathyroidism and hypophosphatemia. When they are prescribed physiological doses of vitamin D, the concentration of l,25-(OH) 2 -D in the serum quickly increases, exceeding the normal level, which indicates stimulation of renal 25-OH-D 3 -lα-hydroxylase and substrate regulation of hormone production. As vitamin D reserves in the body are restored in patients with an initial vitamin D deficiency, the level of l,25-(OH) 2 -D decreases to normal. Similarly, in animals with D-avitaminosis, the synthesis of 1,25-(OH) 2 -D 3 is increased compared to that in animals with sufficient reserves of vitamin D in the body, which is recorded by the conversion of 3 H-25-OH-D 3 in 3 H-l,25-(OH) 2 -D 3 both in vivo and on isolated renal tubules. Moreover, administration of vitamin D or l,25-(OH) 2 -D 3 to animals with D-beri deficiency rapidly reduces 1α-hydroxylase activity in vivo and in vitro. In the mechanism of suppression of 25-OH-D-1α-hydroxylase activity by the hormone (l,25-(OH) 2 -D), inhibition of gene transcription may play a role.
Calcium
IN early studies It has been shown in rats that reducing dietary calcium increases the conversion of 3 H-25-OH-D 3 to 3 H-l,25-(OH) 2 -D 3 . It was then found that calcium deficiency also increases the concentration of l,25-(OH) 2 -D in plasma, and this effect practically disappeared in parathyroidectomized animals. Artificial hypocalcemia or a reduction in the amount of calcium in food increases the plasma level of l,25-(OH) 2 -D in humans, which is associated with an increase in the concentration of parathyroid hormone in the serum. In addition, plasma l,25-(OH)2-D levels are often elevated in patients with primary hyperthyroidism; it also increases after PTH infusions. All these observations indicate that calcium deficiency, either directly or through stimulation of PTH secretion, increases the synthesis of l,25-(OH) 2 -D in the kidneys. When calcium is restricted in the diet, the level of l,25-(OH) 2 -D in the serum increases slightly even in parathyroidectomized animals, but the reduced calcium concentration in the medium does not stimulate the activity of the enzyme that forms l,25-(OH) 2 -D in culture chicken kidney cells. At the same time, the addition of PTH to the medium of cultured kidney cells enhances the synthesis of l,25-(OH) 2 -D, which proves the direct action of parathyroid hormone. Increases or decreases in human serum l,25-(OH)2-D under conditions of calcium deficiency or excess are closely correlated with small changes in serum PTH levels. These results indicate the important role of the Ca - PTH system in the regulation of l,25-(OH) 2 -D levels in human plasma.
Phosphate
Restriction of phosphates in the diet increases the conversion of 3 H-25-OH-D 3 to 3 H-l,25-(OH) 2 -D 3 in animals. Phosphate deficiency also increases the concentration of l,25-(OH) 2 -D in the blood serum of animals and humans, and this effect does not depend on the function of the parathyroid glands. The mechanism of this effect is unknown. It is assumed that it is mediated by a decrease in the content of inorganic phosphorus in P0J kidney cells, but the decrease in phosphate concentration in the medium did not stimulate the synthesis of l,25-(OH) 2 -D by the chicken kidney cell culture. In rats, the increase in plasma l,25-(OH)2-D in response to dietary phosphorus restriction (reflecting renal synthesis of the hormone) is blocked by hypophysectomy, despite persistence of hypophosphatemia. Thus, it is possible that hormonal mediation of stimulation of renal synthesis of 1,25-(OH) 2 -D occurs during phosphorus deficiency.
Other factors
A number of other possible factors regulating renal synthesis of l,25-(OH) 2 -D have also been studied. In young growing animals, children and adolescents, the level of l,25-(OH) 2 -D in plasma is higher than after puberty. The content of 1,25-(OH) 2 -D in plasma also decreases with aging. It was therefore assumed that such effects might be related to growth hormone. Hypophysectomy reduces the level of l,25-(OH) 2 -D in the plasma of rats, and this effect is neutralized by growth hormone (GH). Moreover, administration of GH to naive rats increases the content of l,25-(OH) 2 -D in plasma. On the other hand, administration of GH to children with growth hormone deficiency does not increase serum l,25-(OH) 2 -D levels, despite growth stimulation; the content of l,25-(OH) 2 -D is not always increased during active acromegaly. Thus, the role of GH as a stimulus for the synthesis of l,25-(OH) 2 -D in humans remains unclear. With the same reason, one can assume the participation of other factors that bring the increased absorption of calcium in the intestine under the influence of l,25-(OH) 2 -D into accordance with the needs of the growing skeleton, for which further research is necessary.
Other physiological conditions that trigger the body's need for accelerated absorption of calcium include pregnancy, egg shell formation in birds, and lactation. In women, the level of l,25-(OH) 2 -D in plasma increases as pregnancy progresses. As already noted, 1,25-(OH) 2 -D can be produced by the placenta, which probably accounts for this increase to some extent. Whether pregnancy is accompanied by additional independent stimulation of renal synthesis of l,25-(OH) 2 -D is not known. It should be noted, however, that in birds, estrogens stimulate the renal synthesis of l,25-(OH) 2 -D, and, as is known, the concentration of estrogens in the blood serum increases as pregnancy progresses. The increase in plasma l,25-(OH)2-D levels during pregnancy may be partly due to the increase in serum DSH concentrations observed during this period. During lactation, the content of 1,25-(OH) 2 -D in serum is also increased. There are contradictions regarding the mechanism of this phenomenon. During breastfeeding, prolactin levels increase, and in birds it has been shown that prolactin stimulates the synthesis of l,25-(OH) 2 -D in the kidneys. However, in women with hyperprolactinemic galactorrhea-amenorrhea syndrome, the level of l,25-(OH) 2 -D in plasma is not increased.
It is well known that metabolic acidosis is accompanied by hypercalciuria, which requires assessment of vitamin D metabolism during acidosis. Under conditions of metabolic acidosis, the synthesis of 1,25-(OH) 2 -D in animals with D-avitaminosis is inhibited, but in humans, in the absence of vitamin D deficiency, mild acidosis, although accompanied by increased excretion of calcium in the urine, does not cause changes in the level of l, 25-(OH)2-D in serum. In addition, in humans, metabolic acidosis does not cause visible changes in intestinal calcium absorption. Thus, in the pathogenesis of calciuria characteristic of metabolic acidosis in humans in the absence of vitamin D deficiency, changes in the metabolism of the latter apparently do not play any role.
In a healthy adult, 3 H-l,25-(OH) 2 -D is converted into more polar compounds within 4-6 hours. About 15-20% of it is excreted in the urine in 1 week, mainly in the form of conjugates of these more polar metabolites, while approximately 50% is excreted in the feces over the same time (also in the form of more polar metabolites and conjugates). The rest probably decomposes by oxidation of the side chain. One can assume; that in a healthy person it is the synthesis of 1,25-(OH) 2 -D, and not its rapid metabolism, that is the main determinant of the concentration of the hormone in plasma.
Level regulation 24.25-(OH) 2 — D
The content of 24,25-(OH) 2 -D in plasma with D-avitaminosis in humans naturally decreases to difficult to determine values; this substance appears in the serum only after the reserves of its predecessors, 25-OH-D, are restored in the body. In the absence of vitamin D deficiency, the serum concentration of 24,25-(OH)2-D in humans directly correlates with the concentration of the precursor (25-OH-D). Thus, the synthesis of 24,25-(OH) 2 -D appears to depend mainly on the availability of 25-OH-D. There is no evidence that 24,25-(OH)2-D synthesis is regulated (in the absence of vitamin D deficiency) by calcium, phosphorus, or PTH. As noted above, the question of whether 24,25-(OH) 2 -D is simply an initial breakdown product of vitamin D or whether it has physiological hormonal activity has not yet been resolved.
DISEASES RELATED TO VITAMIND
Elucidation of the metabolism of vitamin D and the processes of its physiological regulation allows us to consistently analyze the results of disruption of the production and action of this vitamin, as well as the results of its excessive consumption or activation of individual stages of its metabolism.
Vitamin deficiencyD: rickets and osteomalacia
Pathognomonic signs of rickets and osteomalacia are skeletal deformation in children, bone pain, fractures, and proximal muscle weakness. Rickets is characterized by impaired maturation and calcification of cartilage and bones, and osteomalacia, which occurs after completion of skeletal growth, is characterized by impaired bone mineralization. In table 13 lists the main disorders of vitamin D metabolism, leading to era deficiency and, consequently, to the pathology of mineral metabolism.
Leather
Since vitamin D synthesis in the skin occurs non-enzymatically, any intrinsic defects in this process are unknown. Insufficient cutaneous synthesis of vitamin D is most likely caused by a lack of solar radiation due to various circumstances. Accordingly, acquired vitamin D deficiency has been observed in the absence of sun exposure, especially among elderly or chronically ill individuals confined to their homes or hospital wards. Vitamin D deficiency is rare in the United States and is likely due to adequate sun exposure. It is also possible that the need for vitamin D is partially covered by multivitamin supplements, which are widespread in the practice of feeding children, and, in addition, by the addition of vitamin D to milk (usually about 400 IU, or 10 mcg of vitamin D 2 per 1 liter). In England, unlike the United States, rickets in children and adolescents belonging to the immigrant population from Asian countries remains an important problem. The mechanisms of occurrence of D-vitaminosis in this population group (they have not been sufficiently studied) may be associated with limited solar exposure. In addition, a role has been suggested for dietary intake of large amounts of grain products containing phytate, which could increase the loss of vitamin D in feces and impair the absorption of calcium in the intestine.
Liver
Genetic deficiency of hepatic 25-hydroxylase has not been described. However, not only 25-OH-D 3, but also DSG is synthesized in the liver. In addition, normal bile formation may be important for the absorption of vitamin D in the intestine and the maintenance of enterohepatic circulation. Therefore, in severe liver diseases and biliary tract sometimes osteomalacia occurs. Possible reason osteomalacia, which often occurs in patients receiving long-term anticonvulsants (phenobarbital and phenytoin), is believed to accelerate the breakdown of vitamin D and its metabolites in the liver due to activation of the microsomal oxidase system. It is also possible that these medicinal substances can directly reduce the absorption of calcium and phosphorus in the intestine. Intestinal diseases can also lead to osteomalacia, both as a result of disruption of the enterohepatic circulation of vitamin D, and as a result of direct disruption of the absorption of calcium and phosphate.
Kidneys
The cause of rickets in some rare cases is a genetic deficiency in the synthesis of l,25-(OH) 2 -D in the kidneys. The existence of such a disorder was initially proven in a purely logical way: by the lack of a therapeutic effect of pharmacological doses of vitamin D or 25-OH-D 3 in those children with bone disease in whom complete recovery occurred with the introduction of physiological doses of l,25-(OH) 2 - D 3 (1.2-- 2.4 nmol/day, or 0.5-1.0 mcg/day). Later, such children were noted to have normal levels 25-OH-D and very low concentrations of l,25-(OH) 2 -D in serum, which indicated a violation of the synthesis of the latter. This defect, inherited as an autosomal recessive trait, is called vitamin D-dependent rickets type I.
Among patients with nephrotic syndrome and normal glomerular filtration rate, symptoms of bone disease are rare, but histologically signs of osteomalacia are detected. Levels of 25-OH-D, l,25-(OH) 2 -D and 24,25-(OH) 2 -D are reduced in these cases, reflecting increased losses of vitamin D and its metabolites in the urine, probably due to similar DSG losses. The molecular weight and isoelectric point of DRG are similar to those of albumin, and this could facilitate its filtration by increasing glomerular permeability. Although in patients with nephrosis, urinary loss of 25-OH-D can reach 10 nmol or more per day, it is still less than the calculated average value normal daily circulation of this compound in healthy people. Therefore, loss of 25-OH-D in urine may not be the only reason for its low serum levels in such patients. Moreover, there is no indication of the occurrence of nephrotic syndrome during progressive damage to the glomeruli, which is extremely often accompanied by osteodystrophy upon the onset of renal failure. It can therefore be assumed that urinary losses of vitamin D and DSH metabolites have little effect on the serum concentration of unbound (free) 25-OH-D or 1,25-(OH) 2 -D, which mainly determines the biological effects of vitamin D .
In some patients with chronic renal failure, especially children, rickets occurs against the background of seemingly normal intake of vitamin D from food. Bone biopsies from adults with chronic kidney disease and a bone disorder called renal osteodystrophy often show not only changes due to secondary hyperparathyroidism, but also varying degrees of osteomalacia. It has also been well known for a long time that in patients with chronic kidney disease, calcium absorption in the intestine is impaired. Almost 40 years ago, it was shown that calcium absorption in the intestine in patients with renal osteodystrophy and severe renal failure averages only 1.8 mmol/day, or only 7% of the amount of calcium contained in the diet, while in healthy people, receiving the same diet providing approximately 27 mmol of calcium per day, absorption reaches 8.1 mmol/day, or 28%. Extremely low absorption of calcium in the intestine in patients with renal osteodystrophy cannot be treated with physiological doses of vitamin D, which cure rickets in patients with vitamin D deficiency. As noted above, calcium absorption in the intestine depends mainly on the concentration of l,25-(OH) 2 -D in the plasma, which in turn should be determined by the ability of the kidneys to synthesize this hormone. Recent observations among adult patients with kidney diseases, found normal or even slightly increased levels of 1,25-(OH) 2 -D in plasma during a period of moderate decrease in glomerular filtration rate (up to approximately 50 mm/min); This is probably due to the development of secondary hyperparathyroidism, characteristic of the early stages of renal failure. In addition, in such patients, calcium absorption in the intestine was usually within normal limits. With the progression of renal failure, the content of l,25-(OH) 2 -D in plasma decreases and reaches very low or undetectable levels in kidney-deprived patients on dialysis. When GFR fell below 50 ml/min, a progressive decrease in calcium absorption in the intestine was observed. There is a report that in children the content of l,25-(OH) 2 -D in plasma can decrease by as much as early stages renal failure. Although frequent damage to tubular-interstitial tissue in children could limit the synthesis of l,25-(OH) 2 -D by the remaining renal tissue due to selective damage to the tubules, a decrease in the level of this compound in plasma is not always detected in such cases.
Oral administration of l,25-(OH) 2 -D to patients with chronic kidney disease enhances the absorption of calcium in the intestine and is directly dependent on the dose. Approximately 1.6 nmol, or 0.68 μg of 1,25-(OH) 2 -D 3 per day (since an amount close to the estimated normal turnover rate increases calcium absorption in the intestine to the level observed in healthy person. Thus, renal failure, apparently, is not accompanied by a significant impairment of the response to 1,25-(OH) 2 -D 3 Long-term administration in chronic renal failure can, therefore, increase the concentration of calcium in the serum and reduce serum levels. levels of PTH and alkaline phosphatase and help eliminate renal osteomalacia. However, in some patients with renal failure and isolated osteomalacia (without biochemical or histological signs of hyperparathyroidism), the administration of 1,25-(OH) 2 -D quickly leads to hypercalcemia, but does not cure bone disease. Although these data suggest a deficiency of not only l,25-(OH) 2 -D, but also some other metabolite of vitamin D in these patients, the results of recent studies indicate that osteomalacia in such cases could be associated with aluminum poisoning. It is not yet known whether the administration of 1,25-(OH) 2 -D 3 and, therefore, maintaining its normal serum concentration along with correction of serum phosphate levels in the early stages of renal failure can prevent the development of renal osteodystrophy as this failure progresses . Since l,25-(OH) 2 -D enhances the absorption of calcium and phosphorus in the intestine, the resulting increase in their serum levels could cause soft tissue calcification and thereby accelerate the development of renal failure.
The concentration of l,25-(OH) 2 -D in serum is reduced or inappropriately increased in certain disorders accompanied by dysregulation of the synthesis of this compound in the kidneys. Low serum l,25-(OH)2-D levels are observed in patients with postoperative or idiopathic hypoparathyroidism. Administration of parathyroid gland extract to such patients normalizes the content of this compound in the serum, which can be explained both by the direct effect of PTH and by concomitant phosphaturia and a decrease in the concentration of phosphate in the serum. In patients with pseudohypoparathyroidism, the serum level of l,25-(OH) 2 -D is also reduced, but it does not increase after administration of parathyroid gland extract. However, administration of dibutyryl-cAMP to patients with pseudohypoparathyroidism type I increases the serum concentration of l,25-(OH) 2 -D. Dibutyryl-cAMP acts, but in all likelihood, due to the “bypass” of the protein defect present in such free animals that couples the interaction of the PTH receptor with adenylate cyclase. A decrease in the level of 1,25-(OH) 2 -D in the blood serum in patients with hypoparathyroidism and pseudohypoparathyroidism, apparently plays a role in the pathogenesis of the resulting hypocalcemia, since its administration can in such cases maintain normal serum calcium concentrations.
The possibility of impaired vitamin D metabolism has also been studied in conditions associated with hypophosphatemia accompanied by rickets or osteomalacia. In patients with X-linked hypophosphatemic rickets, serum concentrations of l,25-(OH)2-D may be lower than in age-matched healthy individuals, suggesting an impairment in the synthesis of this substance in addition to a known underlying defect in phosphate transport. There is an experimental model of X-linked hypophosphatemic rickets in mice, which also exhibit slightly reduced plasma concentrations of l,25-(OH) 2 -D. Moreover, when phosphate is limited in their food, this concentration decreases sharply, while when Ca is limited, it increases. These data support the view that phosphate regulation of 1α-hydroxylase activity is impaired in X-linked hypophosphatemic rickets. The skeletal condition of such patients improves when they are given (along with phosphate supplements) physiological doses of l,25-(OH) 2 -D. Similar dysregulation of renal l,25-(OH)2-D phosphate synthesis may occur in patients with acquired hypophosphatemic osteomalacia, as seen in mesenchymal tumors.
Target tissues
Over the past few years, descriptions of cases of rickets associated with elevated serum levels of l,25-(OH) 2 -D in children have appeared. Some patients, in addition to bone damage, have alopecia and (or) dental abnormalities. Recent studies on cultured skin fibroblasts from such patients have revealed the absence or impairment of l,25-(OH) 2 -D receptors, which are detected in cultured fibroblasts from healthy individuals. These examples thus provide evidence for the existence of a disease that may result from an abnormality of the receptors that mediate the action of vitamin D metabolites.
EXCESS VITAMIN D
Vitamin D toxicity
Excessive intake of vitamin D - 100,000 IU/day or more (over 6.5 µmol, or 2.5 mg/day) is accompanied by hypercalcemia and metastatic calcification of many organs. This pathology appears to result from the direct action of vitamin D or, more likely, 25-OH-D 3 on the target tissue, since the plasma l,25-(OH) 2 -D level remains normal. Hypercalcemia often persists even after vitamin D withdrawal, which is likely due to the long half-life of 25-OH-D, as well as the accumulation of vitamin D in adipose tissue, muscle, and liver. With the therapeutic use of 25-OH-D 3 or l,25-(OH) 2 -D 3 there is also a risk of hypergcalcemia and soft tissue calcification, but when 1,25-(OH) 2 -D 3 is discontinued, it soon disappears due to rapid clearance of this metabolite.
Primary hyperparathyroidism
Hyperparathyroidism is sometimes accompanied by an increase in plasma l,25-(OH)2-D levels, reflecting the stimulatory effect of PTH on the renal synthesis of this compound. With an increase in its concentration in plasma in such patients, calcium absorption in the intestine and calcium excretion in the urine increase. There is evidence that in patients with primary hyperparathyroidism with increased level l,25-(OH) 2 -D in plasma, the frequency of nephrolithiasis as a complication of the underlying disease increases due to more pronounced hypercalciuria. However, there are still contradictions on this issue.
Idiopathic hypercalciuria
In a number of patients with nephrolithiasis, hypercalciuria is recorded in the absence of hypercalcemia. In such cases they speak of idiopathic hypercalciuria. It is known that in these patients the absorption of calcium in the intestine is accelerated, and in recent studies they also show an increase in levels. l,25-(OH) 2 -D in plasma, which probably causes increased calcium absorption. Conflicting opinions remain regarding the mechanism of activation of renal synthesis of l,25-(OH) 2 -D in such patients. Some of them may have a primary defect of calcium loss through the kidneys, as well as mild secondary hyperparathyroidism; in others, the presence of hypophosphatemia and, consequently, some kind of disturbance in phosphate metabolism, leading to activation of l,25-(OH) 2 -D synthesis. The possibility of the existence of other mechanisms for activating its synthesis in patients of this group remains unknown.
Sarcoidosis
Sarcoidosis may be accompanied by hypercalciuria, hypercalcemia and, less commonly, nephrolithiasis or nephrocalcinosis. It has been shown that hypercalciuria and hypercalcemia in sarcoidosis accompany increased absorption in the intestine and an increase in the sensitivity of this process to vitamin D. The level of 1,25-(OH) 2 -D in the serum in such patients may be initially elevated or increase excessively with the administration of vitamin D 2 . Since in healthy people the administration of vitamin D 2 does not increase the content of l,25-(OH) 2 -D in the serum, these observations indicate a dysregulation of the synthesis of the latter in sarcoidosis, in which it becomes dependent on the availability of precursors. A recent report of a kidney-deprived, dialysis patient with sarcoidosis and hypercalcemia whose serum l,25-(OH)2-D was elevated suggests the possibility of extrarenal l,25-(OH)2-D production in sarcoidosis (presumably for sarcoid granuloma).
Make an appointment with an endocrinologist
Dear patients, We provide the opportunity to register directly to see the doctor you want to see for a consultation. Call the number listed at the top of the site, you will get answers to all your questions. First, we recommend that you study the section.
How to make an appointment with a doctor?
1) Call the number 8-863-322-03-16 .
1.1) Or use the call from the site:
Request a call
Call the doctor
1.2) Or use the contact form.


