Insulin Lantus is a hypoglycemic type drug, the use of which is prescribed to insulin-dependent people - for this, the patient must undergo all the necessary studies to make a specific diagnosis. This drug with the active ingredient glargine can be used not only by adults, but also by children over 6 years old.
What is insulin
If your doctor has prescribed a remedy such as a Lantus syringe pen, then first it would be a good idea to find out what insulin actually is. So, insulin is a peptide hormone produced by the pancreas; it has a great effect on metabolism in almost all tissues of the human body. But the main task is to reduce the concentration of glucose in the blood.
Due to the slow absorption after Lantus is injected under the skin, this drug can be used once a day (it is sometimes called extended release). The main function is to regulate carbohydrate metabolism, i.e. glucose metabolism. Glargine, which is part of the drug, is absorbed more slowly compared to NPH insulin, resulting in no peak of activity, which, as a rule, immediately follows the injection.
Insulin - release form
Insulin extended validity is produced in such a form as Lantus syringe pen. The solution intended for injection courses is supplied in a special cartridge containing 3 ml of this product. One blister pack contains 5 such cartridges. 1 ml of solution contains 3.6378 mg (equal to 100 units) insulin glargine and several additional ingredients.
It is recommended to store the drug for no more than 3 years after production. For normal maintenance of Lantus, you need a refrigerator with a temperature within 2-8 degrees. Shelf life at temperature conditions from 15 to 25 degrees decreases to 4 weeks. Freezing the solution is strictly prohibited! When buying a medicine, do not be lazy to check the current expiration date so as not to harm your own health.
Lantus - instructions for use
The drug is intended exclusively for administration under the skin, and therefore it is prohibited to administer it intravenously. The long (extended) period of action is due to the fact that Lantus insulin is injected into the subcutaneous adipose tissue. For each injection, it is recommended to vary the site within the recommended local areas. Intravenous administration can lead to various disorders and the development of severe hypoglycemia. During Lantus therapy, the patient must lead a certain lifestyle.
Before administration, you should carefully read the instructions that come with the syringe pen in order to strictly follow the recommendations of the manufacturer. If the syringe pen or its needle is in a faulty condition, then you need to use a new product. Before attaching the cartridge to the syringe pen, the patient must keep the solution at room temperature for several hours. Cartridges should be used only those in which the drug solution is free of turbidity, color changes and sediment.
Before using the cartridge, be sure to remove all air bubbles - this is clearly stated in the instructions. We should not forget that refilling used cartridges is prohibited, and the product is sold only with a doctor's prescription. Some common analogues of Lantus (in Latin - Lantus) according to active substance and therapeutic effect:
- Levemir Penfill;
- Apidra;
- Glulisine;
- Detemir;
- Humalog mix 25;
- Protafan and others.
How to choose a dose of Lantus
The drug is administered only once a day at the same time. Lantus dosage is selected strictly individually. It is allowed to prescribe the drug to patients diagnosed with diabetes mellitus with a combination of oral antidiabetic drugs. The units of action of Lantus are different compared to the units of action of other insulins - this must be taken into account before you start taking it.
Dose adjustment of the drug may be required if the patient is going to use medications that affect glucose metabolism at the same time. For example, Lantus can potentiate some inhibitors, fibrates, sulfonamides, salicylates, etc. But beta-blockers and clonidine can help both reduce and potentiate (increase) the effectiveness of this drug.
Lantus during pregnancy
Clinical researches pharmacological action The drug has not been tested in pregnant women. Despite this, patient opinions indicate that any negative impact It does not affect the development of the fetus and the course of pregnancy. But Lantus should be prescribed during pregnancy with extreme caution and subject to careful monitoring of the dose used.
Side effects
Lantus, like any other medicine, has its own negative sides. When used, patients often experience effects such as hypoglycemia. This effect is a sign that insulin is being administered in a dose that exceeds the patient's needs. Other side effects Lantus is expressed in:
- the appearance of lipoatrophy, lipohypertrophy in the subcutaneous tissue and skin;
- development of retinopathy, dysgeusia and decreased vision;
- disorders of the sensory organs and nervous system;
- metabolic disorders such as hypoglycemia;
- allergic reactions at the injection site, swelling;
- anaphylactic shock;
- bronchospasm;
- sodium retention in the body;
- myalgia.
Contraindications
Despite the fact that Lantus, judging by many reviews, is able to prolong effective treatment it has several contraindications that you should definitely familiarize yourself with. First of all, the drug should not be used by patients who are intolerant active substance the drug or its auxiliary components. Other contraindications for insulin of this type:
- hypoglycemia;
- treatment of diabetic ketoacidosis;
- age less than 6 years.
In addition, Lantus should be taken with extreme caution by people who have high health risks during episodes of hypoglycemic disease. This also includes patients with proliferative retinopathy, narrowing of cerebral and coronary vessels. At the same time, it is necessary to avoid activities that require increased attention, because With hyper- or hypoglycemia, visual acuity and the ability to concentrate sometimes decrease.

Lantus insulin price
How much does the drug cost today? It is impossible to give an unambiguous answer to this question, because... The cost of Lantus insulin can vary greatly depending on the pharmacy and city. The average price of a medicine of this type is from 3 to 3.5 thousand rubles. But there are places where 3 ml of injection solution costs up to 4 thousand rubles. Therefore, before purchasing, the patient should do a price comparison to choose the most economical option.
Video
Insulin (from lat. insula- islet) is a protein-peptide hormone produced by the β-cells of the islets of Langerhans in the pancreas. Under physiological conditions in β-cells, insulin is formed from preproinsulin, a single-chain precursor protein consisting of 110 amino acid residues. After being transported across the rough endoplasmic reticulum membrane, a 24-amino acid signal peptide is cleaved from preproinsulin to form proinsulin. The long chain of proinsulin in the Golgi apparatus is packaged into granules, where four basic amino acid residues are cleaved by hydrolysis to form insulin and a C-terminal peptide (the physiological function of the C-peptide is unknown).
The insulin molecule consists of two polypeptide chains. One of them contains 21 amino acid residues (chain A), the second - 30 amino acid residues (chain B). The chains are connected by two disulfide bridges. The third disulfide bridge is formed inside chain A. The total molecular weight of the insulin molecule is about 5700. The amino acid sequence of insulin is considered conservative. Most species have one insulin gene, which codes for one protein. The exception is rats and mice (they have two insulin genes), they produce two insulins that differ in two amino acid residues of the B-chain.
The primary structure of insulin in different biological species, incl. and among different mammals, varies somewhat. The closest structure to human insulin is pork insulin, which differs from human insulin in one amino acid (in its B chain, instead of the threonine amino acid residue, it contains an alanine residue). Bovine insulin differs from human insulin in three amino acid residues.
Historical reference. In 1921, Frederick G. Banting and Charles G. Best, working in the laboratory of John J. R. MacLeod at the University of Toronto, isolated an extract from the pancreas (later found to contain amorphous insulin) that lowered blood glucose levels in dogs with experimental diabetes mellitus. In 1922, pancreatic extract was administered to the first patient, 14-year-old Leonard Thompson, a diabetic, and thereby saved his life. In 1923, James B. Collip developed a method for purifying the extract isolated from the pancreas, which later made it possible to obtain from the pancreas of pigs and cattle cattle active extracts that give reproducible results. In 1923, Banting and MacLeod were awarded the Nobel Prize in Physiology or Medicine for the discovery of insulin. In 1926, J. Abel and V. Du Vigneault obtained insulin in crystalline form. In 1939, insulin was first approved by the FDA (Food and Drug Administration). Frederick Sanger completely deciphered the amino acid sequence of insulin (1949-1954) In 1958, Sanger was awarded Nobel Prize for his work on deciphering the structure of proteins, especially insulin. In 1963, artificial insulin was synthesized. The first recombinant human insulin was approved by the FDA in 1982. A rapid-acting insulin analogue (insulin lispro) was approved by the FDA in 1996.
Mechanism of action. In the implementation of the effects of insulin, the leading role is played by its interaction with specific receptors localized on plasma membrane cells, and the formation of the insulin receptor complex. In combination with the insulin receptor, insulin enters the cell, where it affects phosphorylation processes cellular proteins and triggers numerous intracellular reactions.
In mammals, insulin receptors are found on almost all cells - both on classical insulin target cells (hepatocytes, myocytes, lipocytes), and on blood cells, brain and gonads. The number of receptors on different cells ranges from 40 (erythrocytes) to 300 thousand (hepatocytes and lipocytes). The insulin receptor is constantly synthesized and degraded; its half-life is 7-12 hours.
The insulin receptor is a large transmembrane glycoprotein consisting of two α-subunits with a molecular weight of 135 kDa (each containing 719 or 731 amino acid residues, depending on the splicing of the mRNA) and two β-subunits with a molecular weight of 95 kDa (each containing 620 amino acid residues). The subunits are connected to each other by disulfide bonds and form a heterotetrameric β-α-α-β structure. The alpha subunits are located extracellularly and contain insulin-binding sites, being the recognition part of the receptor. Beta subunits form a transmembrane domain, have tyrosine kinase activity and perform signal transduction functions. The binding of insulin to the α-subunits of the insulin receptor leads to stimulation of the tyrosine kinase activity of the β-subunits by autophosphorylation of their tyrosine residues, aggregation of α,β-heterodimers and rapid internalization of hormone-receptor complexes occurs. The activated insulin receptor triggers a cascade of biochemical reactions, incl. phosphorylation of other proteins within the cell. The first of these reactions is the phosphorylation of four proteins called insulin receptor substrates—IRS-1, IRS-2, IRS-3, and IRS-4.
Pharmacological effects of insulin. Insulin affects almost all organs and tissues. However, its main targets are the liver, muscle and fat tissue.
Endogenous insulin is the most important regulator of carbohydrate metabolism, exogenous insulin is a specific sugar-lowering agent. The effect of insulin on carbohydrate metabolism is due to the fact that it enhances the transport of glucose through cell membrane and its utilization by tissues, promotes the conversion of glucose into glycogen in the liver. Insulin, in addition, inhibits endogenous glucose production by suppressing glycogenolysis (the breakdown of glycogen into glucose) and gluconeogenesis (the synthesis of glucose from non-carbohydrate sources - for example, from amino acids, fatty acids). In addition to hypoglycemic, insulin has a number of other effects.
The effect of insulin on fat metabolism is manifested in the inhibition of lipolysis, which leads to a decrease in the flow of free fatty acids into the bloodstream. Insulin prevents the formation of ketone bodies in the body. Insulin enhances the synthesis of fatty acids and their subsequent esterification.
Insulin is involved in protein metabolism: it increases the transport of amino acids across the cell membrane, stimulates the synthesis of peptides, reduces protein consumption by tissues, and inhibits the conversion of amino acids into keto acids.
The action of insulin is accompanied by activation or inhibition of a number of enzymes: glycogen synthetase, pyruvate dehydrogenase, hexokinase are stimulated, lipases are inhibited (both hydrolyzing adipose tissue lipids and lipoprotein lipase, which reduces the “turbidity” of blood serum after eating a fat-rich meal).
In the physiological regulation of the biosynthesis and secretion of insulin by the pancreas, the main role is played by the concentration of glucose in the blood: when its content increases, insulin secretion increases, and when it decreases, it slows down. In addition to glucose, insulin secretion is influenced by electrolytes (especially Ca 2+ ions), amino acids (including leucine and arginine), glucagon, and somatostatin.
Pharmacokinetics. Insulin preparations are administered subcutaneously, intramuscularly or intravenously (only short-acting insulins are administered intravenously and only for diabetic precoma and coma). Insulin suspensions should not be administered intravenously. The temperature of the administered insulin should be at room temperature, because cold insulin is absorbed more slowly. The most optimal method for continuous insulin therapy in clinical practice is subcutaneous administration.
The completeness of absorption and the onset of the effect of insulin depend on the injection site (usually insulin is injected into the abdomen, thighs, buttocks, upper arms), dose (volume of insulin administered), insulin concentration in the drug, etc.
The rate of absorption of insulin into the blood from the site of subcutaneous injection depends on a number of factors - the type of insulin, the injection site, the speed of local blood flow, local muscle activity, the amount of insulin administered (it is recommended to administer no more than 12-16 units of the drug at one site). Insulin enters the blood most quickly from the subcutaneous tissue of the anterior abdominal wall, more slowly - from the shoulder area, the front surface of the thigh, and even more slowly - from subscapular region and buttocks. This is due to the degree of vascularization of the subcutaneous fatty tissue of the listed areas. The action profile of insulin is subject to significant fluctuations as in different people, and for the same person.
In the blood, insulin binds to alpha and beta globulins, normally 5-25%, but binding may increase during treatment due to the appearance of serum antibodies (the production of antibodies to exogenous insulin leads to insulin resistance; when using modern highly purified drugs, insulin resistance rarely occurs ). T1/2 from blood is less than 10 minutes. Most of insulin entering the bloodstream undergoes proteolytic breakdown in the liver and kidneys. Quickly eliminated from the body by the kidneys (60%) and liver (40%); less than 1.5% is excreted unchanged in the urine.
Insulin preparations currently used differ in a number of ways, including by source of origin, duration of action, solution pH (acidic and neutral), presence of preservatives (phenol, cresol, phenol-cresol, methylparaben), insulin concentration - 40, 80, 100, 200, 500 U/ml.
Classification. Insulins are usually classified by origin (bovine, porcine, human, and analogues of human insulin) and duration of action.
Depending on the source of production, there are insulins of animal origin (mainly pork insulin preparations), semi-synthetic human insulin preparations (obtained from pork insulin by enzymatic transformation), and genetically engineered human insulin preparations (DNA recombinant, obtained by genetic engineering).
For medical use Insulin was previously obtained mainly from the pancreas of cattle, then from the pancreas of pigs, given that pork insulin is closer to human insulin. Since bovine insulin, which differs from human insulin in three amino acids, quite often causes allergic reactions, today it is practically not used. Porcine insulin, which differs from human insulin in one amino acid, is less likely to cause allergic reactions. If insulin preparations are not sufficiently purified, there may be impurities (proinsulin, glucagon, somatostatin, proteins, polypeptides) that can cause various adverse reactions. Modern technologies make it possible to obtain purified (mono-peak - chromatographically purified with the release of the “peak” of insulin), highly purified (mono-component) and crystallized insulin preparations. Among insulin preparations of animal origin, preference is given to monopeak insulin obtained from the pancreas of pigs. Insulin obtained using genetic engineering methods fully corresponds to the amino acid composition of human insulin.
Insulin activity is determined by a biological method (by its ability to lower blood glucose levels in rabbits) or by a physicochemical method (by paper electrophoresis or paper chromatography). One action unit, or international unit, is the activity of 0.04082 mg of crystalline insulin. The human pancreas contains up to 8 mg of insulin (approximately 200 units).
Insulin preparations based on their duration of action are divided into short-acting and ultra-short-acting drugs - they imitate the normal physiological secretion of insulin by the pancreas in response to stimulation, intermediate-acting drugs and long-acting drugs - they imitate basal (background) insulin secretion, as well as combined drugs (combine both actions) .
The following groups are distinguished:
(the hypoglycemic effect develops 10-20 minutes after subcutaneous administration, the peak of action is achieved on average after 1-3 hours, the duration of action is 3-5 hours):
Insulin lispro (Humalog);
Insulin aspart (NovoRapid Penfill, NovoRapid FlexPen);
Insulin glulisine (Apidra).
Short-acting insulins(onset of action usually after 30-60 minutes; maximum action after 2-4 hours; duration of action up to 6-8 hours):
Soluble insulin [human genetically engineered] (Actrapid HM, Gensulin R, Rinsulin R, Humulin Regular);
Soluble insulin [human semi-synthetic] (Biogulin R, Humodar R);
Soluble insulin [pork monocomponent] (Actrapid MS, Monodar, Monosulin MK).
Long-acting insulin preparations- include intermediate-acting drugs and long-acting drugs.
(onset after 1.5-2 hours; peak after 3-12 hours; duration 8-12 hours):
Insulin isophane [human genetically engineered] (Biosulin N, Gansulin N, Gensulin N, Insuman Bazal GT, Insuran NPH, Protafan NM, Rinsulin NPH, Humulin NPH);
Insulin-isophane [human semi-synthetic] (Biogulin N, Humodar B);
Insulin-isophane [pork monocomponent] (Monodar B, Protafan MS);
Insulin-zinc suspension compound (Monotard MS).
Long-acting insulins(onset after 4-8 hours; peak after 8-18 hours; total duration 20-30 hours):
Insulin glargine (Lantus);
Insulin detemir (Levemir Penfill, Levemir FlexPen).
Combination insulin preparations(biphasic drugs) (the hypoglycemic effect begins 30 minutes after subcutaneous administration, reaches a maximum after 2-8 hours and lasts until 18-20 hours):
Biphasic insulin [human semi-synthetic] (Biogulin 70/30, Humodar K25);
Biphasic insulin [human genetically engineered] (Gansulin 30R, Gensulin M 30, Insuman Comb 25 GT, Mixtard 30 NM, Humulin M3);
Insulin aspart biphasic (NovoMix 30 Penfill, NovoMix 30 FlexPen).
Ultra-short-acting insulins- analogues of human insulin. It is known that endogenous insulin in pancreatic β-cells, as well as hormone molecules in manufactured short-acting insulin solutions, are polymerized and represent hexamers. When administered subcutaneously, hexameric forms are absorbed slowly and a peak concentration of the hormone in the blood, similar to that in a healthy person after a meal, cannot be created. The first short-acting analogue of insulin, which is absorbed from subcutaneous tissue 3 times faster than human insulin, was insulin lispro. Insulin lispro is a derivative of human insulin obtained by rearranging two amino acid residues in the insulin molecule (lysine and proline at positions 28 and 29 of the B chain). Modification of the insulin molecule disrupts the formation of hexamers and ensures rapid release of the drug into the blood. Almost immediately after subcutaneous administration in tissues, insulin lispro molecules in the form of hexamers quickly dissociate into monomers and enter the blood. Another insulin analog, insulin aspart, was created by replacing proline at position B28 with negatively charged aspartic acid. Like insulin lispro, after subcutaneous administration it also quickly breaks down into monomers. In insulin glulisine, replacing the amino acid asparagine of human insulin at position B3 with lysine and lysine at position B29 with glutamic acid also promotes faster absorption. Rapid-acting insulin analogues can be administered immediately before or after meals.
Short-acting insulins(they are also called soluble) are solutions in a buffer with neutral pH values (6.6-8.0). They are intended for subcutaneous use, less often - intramuscular injection. If necessary, they are also administered intravenously. They have a rapid and relatively short-lived hypoglycemic effect. The effect after subcutaneous injection occurs after 15-20 minutes, reaching a maximum after 2 hours; the total duration of action is approximately 6 hours. They are used mainly in the hospital to establish the dose of insulin required for the patient, as well as when a quick (urgent) effect is required - in diabetic coma and precoma. With intravenous administration, T1/2 is 5 minutes, therefore, in diabetic ketoacidotic coma, insulin is administered intravenously. Short-acting insulin preparations are also used as anabolic agents and are prescribed, as a rule, in small doses (4-8 units 1-2 times a day).
Intermediate-acting insulins less soluble, absorbed more slowly from subcutaneous tissue, as a result of which they have a longer lasting effect. The long-term effect of these drugs is achieved by the presence of a special prolongator - protamine (isophane, protafane, basal) or zinc. The slowdown in insulin absorption in preparations containing insulin-zinc composite suspension is due to the presence of zinc crystals. NPH insulin (neutral protamine Hagedorn, or isophane) is a suspension consisting of insulin and protamine (protamine is a protein isolated from fish milk) in a stoichiometric ratio.
To long-acting insulins insulin glargine is an analogue of human insulin obtained by DNA recombinant technology - the first insulin preparation that does not have a pronounced peak of action. Insulin glargine is produced by two modifications in the insulin molecule: replacing the A chain (asparagine) with glycine at position 21 and adding two arginine residues to the C-terminus of the B chain. The drug is a clear solution with a pH of 4. The acidic pH stabilizes insulin hexamers and ensures long-term and predictable absorption of the drug from the subcutaneous tissue. However, due to its acidic pH, insulin glargine cannot be combined with short-acting insulins, which have a neutral pH. A single dose of insulin glargine provides 24-hour peak-free glycemic control. Most insulin preparations have the so-called “peak” action, observed when the concentration of insulin in the blood reaches its maximum. Insulin glargine does not have a pronounced peak because it is released into the bloodstream at a relatively constant rate.
Long-acting insulin preparations are available in various dosage forms that have a hypoglycemic effect of different durations(from 10 to 36 hours). The prolonged effect allows you to reduce the number of daily injections. They are usually produced in the form of suspensions, administered only subcutaneously or intramuscularly. In diabetic coma and precomatous states, long-acting drugs are not used.
Combination insulin preparations are suspensions consisting of neutral soluble short-acting insulin and isophane insulin (medium-acting) in certain proportions. This combination of insulins of different durations of action in one preparation allows the patient to be spared two injections when using the drugs separately.
Indications. The main indication for the use of insulin is type 1 diabetes mellitus, but in certain conditions it is also prescribed for type 2 diabetes mellitus, incl. with resistance to oral hypoglycemic agents, with severe concomitant diseases, in preparation for surgical interventions, diabetic coma, and diabetes in pregnant women. Short-acting insulins are used not only for diabetes mellitus, but also for some other pathological processes, for example, with general exhaustion (as an anabolic agent), furunculosis, thyrotoxicosis, with stomach diseases (atony, gastroptosis), chronic hepatitis, initial forms cirrhosis of the liver, as well as some mental illness(introduction of large doses of insulin - so-called hypoglycemic coma); it is sometimes used as a component of "polarizing" solutions used to treat acute heart failure.
Insulin is the main specific therapy diabetes mellitus. Treatment of diabetes mellitus is carried out according to specially developed regimens using insulin preparations of different durations of action. The choice of drug depends on the severity and characteristics of the course of the disease, the general condition of the patient and the speed of onset and duration of the hypoglycemic effect of the drug.
All insulin preparations are used subject to mandatory adherence to a limited dietary regimen. energy value food (from 1700 to 3000 kcal).
When determining the dose of insulin, they are guided by the level of glycemia on an empty stomach and during the day, as well as the level of glucosuria during the day. The final dose selection is carried out under the control of reducing hyperglycemia, glucosuria, as well as the general condition of the patient.
Contraindications. Insulin is contraindicated in diseases and conditions associated with hypoglycemia (for example, insulinoma), with acute diseases liver, pancreas, kidneys, stomach ulcers and duodenum, decompensated heart defects, acute coronary insufficiency and some other diseases.
Use during pregnancy. Main by medicinal method Treatment of diabetes mellitus during pregnancy is insulin therapy, which is carried out under close supervision. For type 1 diabetes mellitus, insulin treatment is continued. For type 2 diabetes mellitus, oral hypoglycemic agents are discontinued and diet therapy is carried out.
Gestational diabetes mellitus (pregnant diabetes) is a disorder of carbohydrate metabolism that first appeared during pregnancy. Gestational diabetes mellitus is accompanied by an increased risk of perinatal mortality, the incidence of congenital deformities, as well as the risk of diabetes progression 5-10 years after birth. Treatment of gestational diabetes mellitus begins with diet therapy. If diet therapy is ineffective, insulin is used.
For patients with pre-existing or gestational diabetes mellitus, it is important to maintain adequate regulation of metabolic processes throughout pregnancy. The need for insulin may decrease in the first trimester of pregnancy and increase in the second and third trimesters. During and immediately after childbirth, the need for insulin may decrease sharply (the risk of hypoglycemia increases). In these conditions, careful monitoring of blood glucose levels is essential.
Insulin does not cross the placental barrier. However, maternal IgG antibodies to insulin cross the placenta and are likely to cause hyperglycemia in the fetus by neutralizing its secreted insulin. On the other hand, undesirable dissociation of insulin-antibody complexes can lead to hyperinsulinemia and hypoglycemia in the fetus or newborn. It has been shown that the transition from bovine/porcine insulin preparations to monocomponent preparations is accompanied by a decrease in antibody titer. In this regard, during pregnancy it is recommended to use only human insulin preparations.
Insulin analogues (like other recently developed agents) are used with caution during pregnancy, although there is no reliable evidence of adverse effects. In accordance with the generally accepted recommendations of the FDA (Food and Drug Administration), which determine the possibility of using drugs during pregnancy, insulin preparations for their effect on the fetus belong to category B (reproduction studies in animals did not reveal any adverse effects on the fetus, and adequate and strictly controlled studies in pregnant women women have not been conducted), or category C (animal reproduction studies have revealed an adverse effect on the fetus, and adequate and strictly controlled studies have not been conducted in pregnant women, but the potential benefits associated with the use of the drug in pregnant women may justify its use, despite possible risk). Thus, insulin lispro belongs to class B, and insulin aspart and insulin glargine belong to class C.
Complications of insulin therapy. Hypoglycemia. The introduction of too high doses, as well as a lack of carbohydrate intake from food, can cause an undesirable hypoglycemic state; a hypoglycemic coma may develop with loss of consciousness, convulsions and depression of cardiac activity. Hypoglycemia may also develop due to additional factors that increase insulin sensitivity (eg, adrenal insufficiency, hypopituitarism) or increase tissue glucose uptake (exercise).
TO early symptoms hypoglycemia, which is largely associated with activation of the sympathetic nervous system (adrenergic symptoms) includes tachycardia, cold sweat, trembling, with activation of the parasympathetic system - severe hunger, nausea, and a tingling sensation in the lips and tongue. At the first signs of hypoglycemia, urgent measures are necessary: the patient should drink sweet tea or eat a few lumps of sugar. In hypoglycemic coma, a 40% glucose solution is injected into a vein in an amount of 20-40 ml or more until the patient comes out of the coma (usually no more than 100 ml). Hypoglycemia can also be relieved by intramuscular or subcutaneous administration of glucagon.
Weight gain with insulin therapy is associated with the elimination of glucosuria, an increase in the actual calorie content of food, an increase in appetite and stimulation of lipogenesis under the influence of insulin. Subject to the principles rational nutrition this side effect can be avoided.
The use of modern highly purified hormone preparations (especially genetically engineered preparations of human insulin) relatively rarely leads to the development insulin resistance and phenomena allergies, however, such cases are not excluded. The development of an acute allergic reaction requires immediate desensitizing therapy and drug replacement. If a reaction to bovine/porcine insulin preparations develops, they should be replaced with human insulin preparations. Local and systemic reactions (itching, local or systemic rash, formation of subcutaneous nodules at the injection site) are associated with insufficient purification of insulin from impurities or with the use of bovine or porcine insulin that differs in amino acid sequence from human insulin.
The most common allergic reactions are skin reactions mediated by IgE antibodies. Rarely, systemic allergic reactions and insulin resistance mediated by IgG antibodies are observed.
Visual impairment. Transient refractive errors of the eye occur at the very beginning of insulin therapy and disappear on their own after 2-3 weeks.
Edema. In the first weeks of therapy, transient swelling of the legs also occurs due to fluid retention in the body, the so-called. insulin edema.
Local reactions include lipodystrophy at the site of repeated injections (rare complication). There are lipoatrophy (disappearance of subcutaneous fat deposits) and lipohypertrophy (increased subcutaneous fat deposits). These two states are of different nature. Lipoatrophy, an immunological reaction caused mainly by the administration of poorly purified insulin preparations of animal origin, is practically never encountered at present. Lipohypertrophy also develops when using highly purified preparations of human insulin and can occur when the administration technique is violated (cold preparation, alcohol getting under the skin), as well as due to anabolic local action the drug itself. Lipohypertrophy creates cosmetic defect, which is a problem for patients. In addition, due to this defect, the absorption of the drug is impaired. To prevent the development of lipohypertrophy, it is recommended to constantly change injection sites within the same area, leaving a distance of at least 1 cm between two punctures.
Local reactions such as pain at the injection site may occur.
Interaction. Insulin drugs can be combined with each other. Many drugs can cause hypo- or hyperglycemia, or change the response of a diabetic patient to treatment. The interaction that is possible when insulin is used concomitantly with other drugs should be taken into account. medicines. Alpha adrenergic blockers and beta adrenergic agonists increase the secretion of endogenous insulin and enhance the effect of the drug. The hypoglycemic effect of insulin is enhanced by oral hypoglycemic agents, salicylates, MAO inhibitors (including furazolidone, procarbazine, selegiline), ACE inhibitors, bromocriptine, octreotide, sulfonamides, anabolic steroids (especially oxandrolone, methandienone) and androgens (increase tissue sensitivity to insulin and increase tissue resistance to glucagon, which leads to hypoglycemia, especially in the case of insulin resistance; a reduction in insulin dose may be necessary), somatostatin analogues, guanethidine, disopyramide, clofibrate, ketoconazole, lithium preparations, mebendazole, pentamidine, pyridoxine, propoxyphene, phenylbutazone, fluoxetine, theophylline, fenfluramine , lithium preparations, calcium preparations, tetracyclines. Chloroquine, quinidine, and quinine reduce insulin degradation and may increase blood insulin concentrations and increase the risk of hypoglycemia.
Carbonic anhydrase inhibitors (especially acetazolamide), by stimulating pancreatic β-cells, promote the release of insulin and increase the sensitivity of receptors and tissues to insulin; Although the simultaneous use of these drugs with insulin may increase the hypoglycemic effect, the effect may be unpredictable.
A number of drugs cause hyperglycemia in healthy people and aggravate the course of the disease in patients with diabetes. The hypoglycemic effect of insulin is weakened by: antiretroviral drugs, asparaginase, oral hormonal contraceptives, glucocorticoids, diuretics (thiazide, ethacrynic acid), heparin, H2 receptor antagonists, sulfinpyrazone, tricyclic antidepressants, dobutamine, isoniazid, calcitonin, niacin, sympathomimetics, danazol, clonidine, CCB, diazoxide, morphine, phenytoin, somatotropin, thyroid hormones , phenothiazine derivatives, nicotine, ethanol.
Glucocorticoids and epinephrine have the opposite effect of insulin on peripheral tissues. Thus, long-term use of systemic glucocorticoids can cause hyperglycemia, including diabetes mellitus (steroid diabetes), which can occur in approximately 14% of patients taking systemic corticosteroids for several weeks or with long-term use of topical corticosteroids. Some drugs inhibit insulin secretion directly (phenytoin, clonidine, diltiazem) or by reducing potassium reserves (diuretics). Thyroid hormones speed up insulin metabolism.
The most significant and frequent effects on the action of insulin are beta-blockers, oral hypoglycemic agents, glucocorticoids, ethanol, and salicylates.
Ethanol inhibits gluconeogenesis in the liver. This effect is observed in all people. In this regard, it should be borne in mind that alcohol abuse during insulin therapy can lead to the development of a severe hypoglycemic state. Small amounts of alcohol taken with food usually do not cause problems.
Beta blockers may inhibit insulin secretion, alter carbohydrate metabolism, and increase peripheral insulin resistance, leading to hyperglycemia. However, they may also inhibit the effects of catecholamines on gluconeogenesis and glycogenolysis, which carries a risk of severe hypoglycemic reactions in diabetic patients. Moreover, any of the beta-blockers can mask adrenergic symptoms caused by a decrease in blood glucose levels (including tremor, palpitations), thereby impairing the patient's timely recognition of hypoglycemia. Selective beta 1-blockers (including acebutolol, atenolol, betaxolol, bisoprolol, metoprolol) exhibit these effects to a lesser extent.
NSAIDs and salicylates in high doses inhibit the synthesis of prostaglandin E (which inhibits the secretion of endogenous insulin) and thus enhance basal insulin secretion and increase the sensitivity of pancreatic β-cells to glucose; the hypoglycemic effect during simultaneous use may require adjustment of the dose of NSAIDs or salicylates and/or insulin, especially with prolonged co-use.
Currently, a significant number of insulin preparations are produced, incl. obtained from the pancreas of animals and synthesized using genetic engineering methods. The drugs of choice for insulin therapy are genetically engineered, highly purified human insulins that have minimal antigenicity (immunogenic activity), as well as analogs of human insulin.
Insulin preparations are produced in glass bottles, hermetically sealed with rubber stoppers with aluminum lining, in special so-called. insulin syringes or syringe pens. When using syringe pens, the drugs are contained in special cartridge bottles (penfills).
Intranasal forms of insulin and insulin preparations for oral administration are being developed. When insulin is combined with a detergent and administered as an aerosol to the nasal mucosa, effective plasma levels are achieved as quickly as with an IV bolus. Insulin preparations for intranasal and oral administration are under development or undergoing clinical trials.
To get rid of diabetes in medical practice It is customary to use insulin analogues.
Over time, such medications are becoming increasingly popular among doctors and their patients.
This trend can be explained by:
- fairly high efficiency of industrially produced insulin;
- excellent high safety profile;
- ease of use;
- the ability to synchronize drug injections with the hormone’s own secretion.
After some time, patients with type 2 diabetes are forced to switch from pills that lower blood sugar to injections of the hormone insulin. Therefore, the question of choosing the optimal drug is a priority for them.
Features of modern insulins
There are some limitations in the use of human insulin, for example, a slow onset of action (diabetics must inject 30-40 minutes before meals) and too long an operating time (up to 12 hours), which can lead to delayed hypoglycemia.
At the end of the last century, there was a need to develop analogues of insulin that would be devoid of these disadvantages. Short-acting insulins began to be produced with a maximum reduction in half-life.
This brought them closer to the properties of native insulin, which can be inactivated within 4-5 minutes after entering the bloodstream.
Peak-free insulin options can be absorbed evenly and smoothly from subcutaneous fat and do not provoke nocturnal hypoglycemia.
IN last years There was a significant breakthrough in pharmacology, as it was noted:
- transition from acidic solutions to neutral ones;
- production of human insulin using recombinant DNA technology;
- creation of high-quality insulin substitutes with new pharmacological properties.
Insulin analogues alter the timing of action of the human hormone to provide a personalized physiological approach to therapy and maximum convenience for the diabetic.
The drugs make it possible to achieve an optimal balance between the risks of changes in blood sugar levels and achieving the target glycemic level.
Modern analogues of insulin are usually divided into:
- ultra-short (Humalog, Apidra, Penfill);
- prolonged (Lantus, Levemir Penfill).
In addition, there are combined substitute preparations, which are a mixture of ultra-short and long-acting hormones in a certain ratio: Penfill, Humalog mix 25.
Humalog (lispro)
In the structure of this insulin, the position of proline and lysine was changed. The difference between the drug and soluble human insulin is the weak spontaneity of intermolecular associations. Because of this, lispro can be absorbed into the bloodstream of a diabetic more quickly.
If you inject drugs in the same dosage and at the same time, then Humalog will give a peak 2 times faster. This hormone is eliminated much faster and after 4 hours its concentration returns to its original level. The concentration of human simple insulin will be maintained within 6 hours.
Comparing lispro with short-acting simple insulin, we can say that the former can suppress the production of glucose by the liver much more strongly.
There is another advantage of Humalog - it is more predictable and can facilitate the period of adaptation of the dosage to the nutritional load. It is characterized by the absence of changes in the duration of exposure from an increase in the volume of the administered substance.
With simple human insulin, how long it lasts may vary depending on the dose. This is what gives rise to the average duration of 6 to 12 hours.
With an increase in the dosage of Humalog insulin, the duration of its work remains almost at the same level and will be equal to 5 hours.
This suggests that the risk of delayed hypoglycemia does not increase with increasing doses of lispro.
Aspart (Novorapid Penfill)
This insulin analogue is capable of almost perfectly simulating an adequate insulin response to food intake. Its short duration of action causes a relatively weak effect between meals, which makes it possible to obtain the most complete control over blood sugar.
If we compare the result of treatment with insulin analogues with regular short-acting human insulin, a significant increase in the quality of control of postprandial blood sugar levels will be noted.
Combined treatment with Detemir and Aspart makes it possible to:
- almost 100% normalize the daily profile of the hormone insulin;
- qualitatively improve the level of glycosylated hemoglobin;
- significantly reduce the likelihood of developing hypoglycemic conditions;
- reduce the number of amplitudes and peaks in the blood sugar concentration of a diabetic.
It is noteworthy that during therapy with basal-bolus insulin analogues, the average increase in body weight was significantly lower than during the entire period of dynamic observation.
Glulisine (Apidra)
The human insulin analogue Apidra is an ultra-short-acting drug. In terms of its pharmacokinetic, pharmacodynamic characteristics and bioavailability, Glulisine is equivalent to Humalog. In terms of its mitogenic and metabolic activity, the hormone is no different from simple human insulin. Thanks to this, it is possible to use it for a long time, and it is absolutely safe.
As a rule, Apidra should be used in combination with:
- long-acting human insulin;
- analogue of basal insulin.
In addition, the drug is characterized by a faster onset of work and a shorter duration than that of a regular human hormone. It allows diabetic patients to be more flexible in how they use it during meals than the human hormone. Insulin begins its effect immediately after administration, and blood sugar levels drop 10-20 minutes after Apidra was administered subcutaneously.
To avoid hypoglycemia in elderly patients, doctors recommend administering the drug immediately after a meal or simultaneously with it. The reduced duration of action of the hormone helps to avoid the so-called “overlap” effect, which makes it possible to prevent hypoglycemia.
It can be effective for those who are overweight, because its use does not cause further weight gain. The drug is characterized by a rapid onset of maximum concentration compared to other types of hormones regular and lispro.
Apidra is ideal for those suffering from varying degrees of excess weight due to its high flexibility in use. In visceral obesity, the rate of drug absorption may vary, complicating prandial glycemic control.
Detemir (Levemir Penfill)
Levemir Penfill is an analogue of human insulin. It has an average operating time and has no peaks. This helps ensure basal glycemic control throughout the day, but only if used twice.
When administered subcutaneously, Detemir forms substances that bind to serum albumin in the interstitial fluid. After transport through the capillary wall, insulin rebinds with albumin in the bloodstream.
In the preparation, only the free fraction is biologically active. Therefore, binding to albumin and its slow breakdown ensures long-lasting and peak-free operation.
Insulin Levemir Penfill acts smoothly on the body of a diabetic patient and replenishes his full need for basal insulin. It does not require shaking before subcutaneous administration.
Glargine (Lantus)
The insulin substitute Glargine acts ultra-fast. This drug can be well and completely soluble in a slightly acidic environment, but poorly soluble in a neutral environment (in subcutaneous fat tissue).
 Immediately after subcutaneous administration, Glargine enters into a neutralization reaction with the formation of microprecipitation, which is necessary for the further release of hexamers of the drug and their cleavage into monomers and dimers of the hormone insulin.
Immediately after subcutaneous administration, Glargine enters into a neutralization reaction with the formation of microprecipitation, which is necessary for the further release of hexamers of the drug and their cleavage into monomers and dimers of the hormone insulin.
Thanks to the smooth and gradual entry of Lantus into the bloodstream of a patient with diabetes, its circulation occurs within 24 hours. This makes it possible to inject insulin analogues only once a day.
By adding a small amount of zinc, Lantus insulin crystallizes in the subcutaneous layer of fiber, which further lengthens its absorption time. Absolutely all of the mentioned qualities of this drug guarantee its smooth and completely peak-free profile.
Glargine begins to work 60 minutes after subcutaneous injection. Its stable concentration in the patient’s blood plasma can be observed 2-4 hours after the first dose was administered.
Regardless of the exact time of injection of this ultra-fast drug (morning or evening) and the immediate injection site (stomach, arm, leg), the duration of exposure to the body will be:
- average – 24 hours;
- maximum – 29 hours.
Insulin replacement glargine may be fully compatible physiological hormone due to its high efficiency, because the drug:
- qualitatively stimulates sugar consumption by insulin-dependent peripheral tissues (especially fat and muscle);
- inhibits gluconeogenesis (reduces).
In addition, the drug significantly suppresses the process of breakdown of adipose tissue (lipolysis) and protein decomposition (proteolysis), while enhancing the production of muscle tissue.
Medical studies of the pharmacokinetics of Glargine have shown that the peak-free distribution of this drug makes it possible to almost 100% imitate the basal production of the endogenous hormone insulin within 24 hours. At the same time, the likelihood of developing hypoglycemic conditions and sudden jumps in blood sugar levels is significantly reduced.
Humalog mix 25
This drug is a mixture that consists of:
- 75% protaminized suspension of the hormone lispro;
- 25% insulin Humalog.
This and other insulin analogues are also combined according to the mechanism of their release. The excellent longevity of the drug is ensured due to the effect of a protaminized suspension of the hormone lispro, which makes it possible to repeat the basal production of the hormone.
The remaining 25% of insulin lispro is a component with an ultra-short action period, which has a positive effect on glycemia after meals.
It is noteworthy that Humalog as part of the mixture affects the body much faster in comparison with the short hormone. It provides maximum control of postradial glycemia and therefore its profile is more physiological when compared with short-acting insulin.
Combined insulins are especially recommended for people suffering from type 2 diabetes. This group includes elderly patients who, as a rule, suffer from memory problems. That is why the administration of the hormone before or immediately after meals is very helpful in significantly improving the quality of life of such patients.
Diabetic Health Research age group from 60 to 80 years old with the use of the drug Humalog Mix 25 showed that it was possible to obtain excellent compensation for carbohydrate metabolism. By administering the hormone before and after meals, doctors were able to achieve slight weight gain and an extremely low number of hypoglycemia.
Which insulin is better?
If we compare the pharmacokinetics of the drugs in question, then their prescription by the attending physician is quite justified for diabetes mellitus, both type 1 and type 2. A significant difference between these insulins is the absence of weight gain during treatment and a decrease in the number of nighttime changes in blood glucose concentrations.
In addition, it is important to note the need for only a single administration during the day, which is much more convenient for patients. The effectiveness of the human insulin analogue Glargine in combination with metformin is especially high for patients with type 2 diabetes. Studies have shown a significant reduction in nighttime spikes in sugar concentrations. This helps to reliably normalize daily glycemia.
Page 3 of 14
3. GENERAL INFORMATION ABOUT INSULIN ANALOGUES
3.1.RATIONALE FOR THERAPY WITH INSULIN ANALOGUES
It is well known that proper glycemic control delays the onset and slows the progression of late complications diabetes in adults and young patients with type 1 and type 2 diabetes. 4" 5" 9. This can be achieved with insulin therapy, but soluble human insulins, including biphasic human insulin (BHI), should be administered at least 20-30 minutes before meals, which most patients do not do. At the same time, ultra-short-acting insulin analogues are absorbed much faster than regular insulin; they can be administered immediately before meals or even immediately after meals, which makes it possible to get closer to physiological norm closer than with traditional soluble human insulins.
3.2. NOVORAPID® - ANALOGUE OF FAST-ACTING INSULIN
Insulin analogues are chemically engineered versions of natural insulin. They are designed to more effectively maintain insulin levels that meet physiological needs, thereby reducing some of the limitations inherent in conventional insulin preparations. Insulin aspart (NovoRapid®) is an original analogue of ultra-short-acting insulin, which has a structure almost similar to natural insulin, differing only in one modification - the proline residue at position 28 of the amino acid chain is replaced by an aspartic acid residue (Fig. 1). This replacement reduces the self-aggregation of insulin molecules into dimers and hexamers, which accelerates its absorption. In this case, the interaction of insulin with receptors does not change (Fig. 2, page 6) 10 The modified insulin molecule completely retains the biological hypoglycemic properties of natural insulin, while being absorbed faster and having the expected pharmacokinetics 11 .
Rice. 1. NovoRapid® (insulin aspart) - molecular structure.

Rice. 2. Scheme of the processes occurring in the subcutaneous tissue.
The hexameric structures of the ultra-short-acting insulin analog are broken down into monomers much faster than the hexamers of soluble traditional insulin, which causes their faster absorption from injection sites and entry into the bloodstream.
The ultra-short-acting insulin analogue NovoRapid® is absorbed more quickly from injection sites than soluble human insulin, reaches a higher peak concentration in a shorter time 12 and has a shorter half-life (Fig. 3). Due to this, its onset of action occurs faster (10-20 minutes after injection), and the duration of action is shorter than that of soluble insulin (3-5 hours compared to 6-8 hours).
These differences determine the following three advantages of NovoRapid® compared to soluble insulin:
• it can be administered immediately before meals, and not 30 minutes before meals;
• a short period of action creates convenience for the patient, who now does not have to take additional food in the intervals between main meals;
• administration immediately before meals, short duration of action potentially reduces the risk of hypoglycemia and does not require glycemic control 13" 14.
Special clinical studies showed that switching 850 adult patients with type 1 diabetes from soluble insulin to insulin aspart administered before meals reduced the incidence of nocturnal hypoglycemia by 50% (p = 0.005), while the HbA1c level significantly decreased by 0.12- 0.15%. Recent studies have shown a reduction in the incidence of hypoglycemia by more than 70% in patients with type 1 diabetes treated with NovoRapid 15 .
Administration of NovoRapid® immediately before meals provides adequate control of postprandial glycemia and glycemia during the day, and also creates convenience for patients.
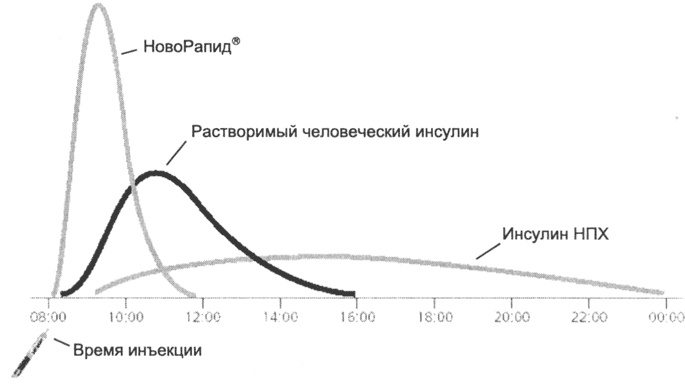
Figure 3:. Scheme of the kinetics of various insulins.
3.3. NOVOMIX® 30 - ANALOGUE OF BIPHASE INSULIN
NovoMix® 30 (biphasic insulin aspart) consists of 30% ultra-short-acting insulin analogue aspart and 70% crystalline protamine insulin aspart. In biphasic preparations, rapid absorption of the soluble component is maintained, which ensures a rapid onset of action and rapid elimination 16 . Slow absorption of the protaminated form (similar to NPH insulin) is a component of basal insulin therapy. As a result, biphasic insulin provides both basal and meal-related insulin requirements (Figure 4).
3.3.1. Benefits of biphasic insulins
Biphasic (premixed) insulins allow patients to maintain an insulin schedule that includes fast-acting and intermediate-acting components (which is necessary to satisfy both basal and meal-related needs), while reducing the number of daily injections. The use of ultra-short-acting insulin analogues makes it possible to more clearly separate the satisfaction of basal and meal-related insulin needs (the “dual absorption” concept of biphasic insulin aspart). This provides the opportunity to significantly improve postprandial glycemic control, and also has other advantages of fast-acting analogues, for example, the absence of the need to observe an interval between injection and meal. The administration schedule for biphasic insulins is the simplest of all possible. In addition, the use of biphasic insulins allows the patient to abandon vials and syringes and switch to modern systems administration of drugs. The use of biphasic insulin eliminates the need to mix different drugs, which not only simplifies the administration process, but also prevents risk possible errors when mixing.
When using biphasic insulin, there is no need for large quantities injections, when fast-acting insulin and an intermediate-acting drug are administered at the same time. Although the use of biphasic insulin limits the possibilities for individual dosing, there is no reason to believe that self-mixing is more effective in improving glycemic control in sick prepubertal children 12" 17. In biphasic insulin preparations, the ratio of rapid-acting insulin to intermediate-acting insulin is fixed. The most common ratio is 30% analogue ultra-short action and 70% average duration of action is suitable for many patients.
Biphasic insulins are widely used by diabetic patients, including children and adolescents, throughout the world. A study of 2873 children from Europe, Japan and North America found that 60% (n = 1707) received two injections per day. From total number Of patients receiving two or three injections, 37% used premixed insulins alone or in combination with another insulin 18 .
The use of biphasic drugs is advisable for children and adolescents, for whom a reduction in the number of injections and a free lifestyle are priorities. It is quite difficult for parents and teachers to control the administration of injections by children during school years. With the onset of adolescence, when personal freedom, autonomy and acceptance become the leading factors, the need to perform multiple injections throughout the day can lead to feelings of resentment and rebellion, with some patients simply skipping insulin injections. If treatment limits leisure activities and disrupts eating habits, certain compromises must be made. Children early age, patients with learning difficulties, as well as those who have difficulty adapting to a more complex regimen or those whose treatment reduces their quality of life should appreciate the ease of use of biphasic insulins.
Biphasic insulin preparations, which include soluble human insulin, have the disadvantages characteristic of soluble insulin: the need for administration 30 minutes before meals, a time profile of action that does not sufficiently correspond to the physiological secretion of insulin, a high risk of hypoglycemia. At the same time, biphasic analogues, for example NovoMix® 30, do not have these disadvantages and enable many patients to achieve adequate glycemic control with only two injections per day.
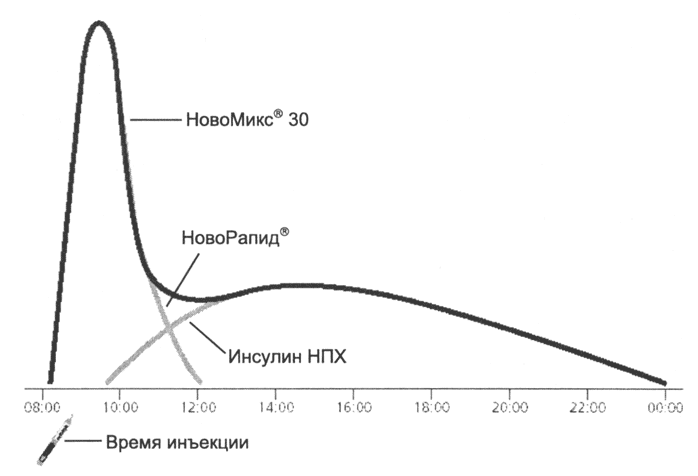
Rice. 4. Double profile of insulin concentration in the blood after injection of NovoMix® 30.
Indeed, these simple and convenient treatment regimens achieve effective metabolic control in many patients, compared with more complex (and theoretically more effective) injection regimens. In adults, the dual absorption profile of NovoMix® 30 compensates for basal and prandial insulin needs more accurately than DAYS 16-19-20. In addition, NovoMix® 30 reduces both total and postprandial blood glucose concentrations to a greater extent than DNI 1 "20 23. Data on the treatment of children with NovoMix® 30, as well as other biphasic insulin analogues, are limited, but at present A number of studies are being conducted on the use of the drug NovoMix® 30 in pediatrics. Biphasic insulin analogues can be used instead of conventional biphasic insulins in all cases where the latter are currently used.
NovoMix® 30, a biphasic insulin analog, creates a biphasic insulin concentration profile that is more physiological than conventional biphasic insulin, separated into a meal-related peak and a basal component.
3.4. ANALOGUES LONG-ACTING
There is currently one long-acting insulin analogue product on the market (insulin glargine) and others, including insulin detemir, are in development. It is hoped that in the future, with the help of long-acting analogues, it will be possible to overcome the disadvantages of conventional long-acting insulins, such as the less predictable profile of zinc insulins (Ultralente, Monotard). Currently, data on the effectiveness and safety of long-acting insulin analogues remain limited.
3.5. SAFETY OF INSULIN ANALOGUES
Safety issues should be discussed before initiating treatment with insulin analogues. Novo Nordisk was the first to work towards creating insulin analogues with desired pharmacokinetic properties. Novo Nordisk's early research into insulin analogues in the 1980s included the development of B10Asp. However, the high affinity for insulin-like growth factor 1 (IGF-1) receptors and the high mitogenetic potential of B10Asp have forced researchers to focus their efforts on the development and search for insulin analogues that would have safer characteristics. This is how the ultra-short-acting insulin analogue aspart (NovoRapid®) was created.
Preclinical and clinical researches insulin analogue aspart indicate that it is equivalent in effectiveness to human insulin, with the same low likelihood of adverse or unusual biological effects. This is confirmed by the comparative data presented in Fig. 5.
Rice. 5. Relative affinity for IGF-1 receptors and mitogenetic potential of some insulin analogues.


